Introduction
Among the various types of cancers affecting the body, some of them are caused by environmental stimuli and life style practices, which can be modified to halt their progression or to prevent their occurrence. One among them is Squamous cell carcinoma (SCC) which occurs most commonly due to life style habits such as smoking and betel nut chewing.1, 2 SCCs can arise in various sites such as uterine cervix, oesophagus, anal canal and head and neck region.3, 4, 5, 6, 7 SCCs of Head and Neck region(HNSCCs) constitute the 6th leading cancer in the world, accounting for a huge burden of cancer associated deaths worldwide. This necessitates the study of HNSCCs and their associated risk factors, so that the morbidity and mortality can be curtailed. The advent of increasing evidence based therapy in medicine today aided by advancing research in molecular pathogenesis of cancers has led us to a new era of targeted chemotherapy for the cancers.8 Recently, various studies are identifying novel markers representing molecular pathways in Squamous cell carcinomas of the head and neck region. 8, 9, 10 Among them, the over expression of Cyclin D1, a cell cycle regulator, is documented in the HNSCCs.1 Cyclin D1 correlates well with other pathological parameters in the prognostication of HNSCCs.11, 12, 13, 14 Hence this study is conducted on HNSCCs to study the immunopositivity of Cyclin D1 and to correlate its expression pattern with various clinico-pathological parameters to guide the management and prognostication of these patients.
Aims & Objectives
To study the expression of Cyclin D1in Head and Neck Squamous Cell Carcinomas by immunohistochemistry.
To correlate the expression of Cyclin D1 with histopathological grading and other variables such as gender, age, anatomical site, smoking history, alcohol consumption history and stage of cancer (wherever available).
Materials and Methods
The present study was conducted in the Department of Pathology, in a tertiary care institute at Bangalore, India. It was a retro-prospective study from the period of January 2012 to April 2015 comprising of samples from 150 patients. Institutional ethical clearance was obtained prior to the commencement of the study.
Inclusion criteria
All cases diagnosed as primary squamous cell carcinoma on routine biopsy of head and neck lesions irrespective of age and gender of patient.
Exclusion criteria
Head and neck lesions diagnosed as carcinoma in situ and those cases of squamous cell carcinoma deposits in Lymph nodes of Head and Neck region were excluded from study.
Sample collection
Biopsy and tissue samples of patients submitted for histopathological examination, diagnosed as Squamous cell carcinomas of the Head and Neck region were evaluated. Paraffin embedded samples which were diagnosed as HNSCCs and their corresponding Haematoxylin and Eosin stained sections were retrieved from the archives of pathology department were used for retrospective analysis.
Sample processing
Biopsies and tissue samples were fixed in 10% Neutral buffered formalin solution for an average period of 24 hours followed by processing using an automated histokinette and an automated microtome. Sections were preliminarily stained by routine Haematoxylin and Eosin (H&E) stain and graded according to descriptive Broder’s criteria as well differentiated, moderately differentiated and poorly differentiated tumours. Immunohistochemical (IHC) staining for Cyclin D1 was carried out by peroxidase–antiperoxidase method. Monoclonal antibodies were obtained from Biogenex and Scytek laboratories. Diaminobenzidine (DAB) was used as chromogen. Staining in the basal and suprabasal layer of the normal stratified squamous epithelium in the sections were taken as positive control. Negative control was taken by staining those sections by omitting the primary antibodies. Labelling index percent for Cyclin D1 [Positivity Index -PoI] was calculated by reporting the number of positive cells (nuclei) to the total number of cells (positive and negative), with the result expressed in percentage. A minimum of 500 cells were counted under 40X objective (High power objective). For the immunoquantification of Cyclin D, a semi quantitative evaluation system consisting of three levels: <10%, 10-50%, and >50% was used. The histological grade on the H&E sections and the expression of Cyclin D1were correlated along with other clinical parameters.
Results
All 150 cases were studied histopathologically, evaluated for immunoexpression of Cyclin D1 and correlated with clinical parameters. The age of patients ranged from 25 to 83 years with a mean age of 56 years. Maximum number of cases were seen in the age group of 51 to 60 years (32%). Considering distribution of cases with respect to gender, 108(72%) cases were males and 42 (28%) were females. Male to female ratio was 2.6:1. One hundred and four cases had a history of smoking, accounting for 69.3% of total number of cases with all of them being males (100%). Among the category of non-smokers (46 cases, 31%), 4 were males and 42 were females. Of the 104 cases that had a history of smoking, maximum number of patients (46 cases, 44%) had a history of smoking for a period of 21 to 30 years. Twenty percent of patients had a history of smoking for more than 30 years. One hundred cases (66.6%) gave a positive history of alcohol consumption, all of them were males. Of the non-alcoholics, 81% of them constituted females. Eighty-eight cases had a positive history of tobacco chewing accounting for 58.6% of cases and there was an almost equal distribution between both the genders. Table 1. (Distribution of cases with respect to Demographic variables).
Majority of the cases (59 cases, 39.3%) were seen in the oral cavity, with buccal mucosa (33.8%) being the commonest site, followed by the tongue (30.5%). Among 30.6% of pharyngeal Squamous cell carcinoma, the pyriform fossa was found to be the most commonly involved site (39.1%). Supraglottic SCC was the most frequently encountered laryngeal SCC (40%). Laterality was not considered in the assessment of site as laterality is not applicable in most of these unpaired organs of head and neck region. Table 2: Site distribution of HNSCCs.
Well-differentiated type was the predominant histologic grade accounting for 67 cases (45%), followed closely by moderately differentiated type (63 cases, 42%) and then by poorly differentiated type (20 cases, 13%). Immunopositivity of Cyclin D1 in >50% of tumour cells was seen in a majority of 67 cases (44.7%), followed by 45 cases (30%) showing ≤10% immunostained cells. The remaining 38 cases (25.3%) showed intermediate score of 11-50% of immunopositivity. Out of 67 well differentiated Squamous cell carcinomas, majority [30 cases (44.7%)] of cases showed Cyclin D1 positivity index of ≤10%. Among moderately differentiated type, 33 cases (52.3%) showed Cyclin D1 positivity index of > 50%. Eighty-five percent (17 out of 20 cases) of poorly differentiated type of Squamous cell carcinoma showed Cyclin D1 positivity index of > 50%. The correlation of Cyclin D1 immunoexpression was found to be statistically significant with respect to the various histopathological grades of HNSCC (p=<0.001). There was no significant association between immunoexpression of Cyclin D1 and age, gender, history of smoking, the duration of smoking, alcohol consumption and tobacco chewing. There was no significant differing immunoexpression of Cyclin D1 in the various major anatomical sites of HNSCC. The positivity index was found to be distributed similarly in different anatomical sites. In only twenty-six of the 150 study cases, radical neck dissection was performed. Of these 19 cases (73%) showed metastatic deposits in the lymph nodes. The study of Cyclin D1 immunoexpression in these 19 cases showed a majority of them i.e., 13 cases (68.6%) with > 50% immunoreactive cells. The increasing immunoexpression of Cyclin D1in lymph node positive HNSCC was found to be statistically significant (p= 0.005). Of the 26 cases in which pathological staging could be assessed, immunopositivity of cyclin D1 was found not to be significantly associated with any of the stages(p=0.222). Table 3: Correlation of Cyclin D1 with respect to various parameters.
Table 1
Distribution of cases with respect to Demographic variables
Table 2
Site distribution of HNSCCs
Figure 1
Gross photograph of Squamous cell carcinoma of Lip, B: Gross photograph of Squamous cell carcinoma of Right Cheek

Table 3
Correlation of Cyclin D1 with respect to various parameters
Figure 2
Microphotograph showing keratin pearls in well differentiated squamous cell carcinoma [H&E 10x10]; B: Microphotograph showing moderately differentiated squamous cell carcinoma (H&E-10x40); C: Microphotograph showing poorly differentiated squamous cell carcinoma (H&E-10x40); D: Microphotograph showing nuclear immuno-expression of Cyclin D1 in well differentiated squamous cell carcinoma (score-<10%) (IHC, 10x40); E: Microphotograph showing nuclear immuno-expression of Cyclin D1 in moderately differentiated squamous cell carcinoma (score 45%) (IHC, 10x40); F: Microphotograph showing nuclear immuno-expression of Cyclin D1 in poorly differentiated squamous cell carcinoma (score-85%)(IHC,10x40)
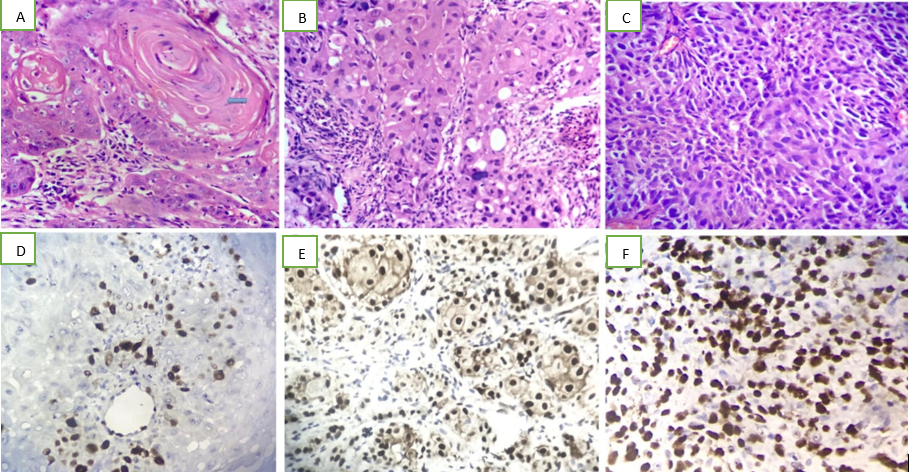
Discussion
The mean age of patients including both men and women in our study of 150 cases was found to be 56 years. This is well comparable with other studies as shown by Vicente et al who found that the mean patient age was 56.6 years and Yu et al whose patients had a mean age of 61.5 years. 13, 15 The present study found no significant correlation of Cyclin D1[p=0.924] expression with age. This is supported by the findings from other studies such as Zhang et al. 16 [Cyclin D1 (p=0.559)]. Various studies conducted by researchers show significant increase in HNSCC in male gender, a similar male preponderance was seen in present study too and there was no significant correlation of Cyclin D1 (p=0.348) with Gender. This is in concordance with authors like Vicente et al and Zhang et al. 13, 16
Authors like Zhang et al and Huang et al who have studied large number of cases have established that majority of their cases had a positive smoking history. 16, 17 Likewise, 69.3% of our cases also proved to be smokers with a long and continuous smoking history. Various authors such as Vicente et al and Zhang et al found no significant association of Cyclin D1 with smoking history. The present study is in agreement with these findings with no significant association of Cyclin D1 [p=0.277] with smoking history. Studies conducted by various authors indicate that alcohol consumption plays a contributory role in the etio-pathogenesis of HNSCC. Kaminagakura et al studied 90 cases and found that 67.7% of them had a significant alcohol consumption history. 18 The present study also found that two thirds of its study patients (67%) admitted to having a significant history of alcohol consumption but found no significant correlation of Cyclin D1 (p=0.393) with alcohol consumption history in concordance with the findings of Vicente et al and Zhang et al.13, 16 The present study highlights that 58.6% of the cases had a positive history of tobacco chewing in concordance with other authors.
The major anatomical sites in the head and neck region affected by SCC are the oral cavity, pharynx and the larynx, followed by other sites like skin of external ear, scalp and the nasal region. Michalides et al, who studied all the head and neck SCCs found the hypopharynx to be most frequently involved site.19 Ashraf et al found the glottic region to be the most often affected in the laryngeal SCCs. 20 The present study which included 150 cases of HNSCCs involving all the major anatomical sites found that majority of cases (39.3%) clustered in the oral cavity, followed by the pharynx and lastly by the larynx. However, present study found no significant correlation of Cyclin D1 with major anatomical sites. This is supported by the findings from other studies too such as Vicente et al, Zhang et al and Raju et al.13, 16, 21
Histopathological grade has proved to be of independent prognostic value.3 The studies conducted by various authors have used modified Broder’s Grading system which sub-classifies SCC into well differentiated, moderately differentiated and poorly differentiated tumour grades. 11, 13, 18, 20, 22 Different studies conducted among patients from variable geographic areas show differing distribution of tumour grades. The present study found a majority of cases (45%) showing a well differentiation pattern, and the least number of cases (13%) to be poorly differentiated. This is in agreement with Vicente et al, Ashraf et al, Satya Das et al, and Choudhary et al.13, 20, 22, 23 As evidenced by the various international studies conducted by the above authors, increasing Cyclin D1 immunoexpression is seen in increasing tumour grade. Similar patterns are also observed in the present study with 85% of poorly differentiated tumours showing Cyclin D1 over-expression.
Huang et al studied 264 SCCs and found that Cyclin D1 overexpression was significantly associated with regional lymph node metastasis (p=0.002).17 In the present study, of the 19 cases which showed regional lymph node metastasis, 68.6% of the cases showed over expression of Cyclin D1. This increasing immunoexpression of Cyclin D1 in cases presenting with nodal metastasis was found to be statistically significant. Cyclin D1 over expression did not correlate well with tumour stage. This is in contrast with the studies done by other authors who obtained positive correlation of Cyclin D1 with respect to advanced tumour stage.14, 16, 24
Conclusion
The immunoexpression of Cyclin D1 is found to be increasingly present in poorer grades of tumour differentiation and in metastatic lymph node deposits. Hence “Cyclin D1 immunoexpression can prove to be of tremendous beneficial value in the early assessment of neoplastic behaviour in head and neck squamous cell carcinomas and may serve as an independent prognostic marker with therapeutic implications.”
