- Visibility 126 Views
- Downloads 14 Downloads
- DOI 10.18231/j.jdpo.2021.001
-
CrossMark
- Citation
A Comparative study for the assessment of diagnostic efficacy and accuracy of various techniques in detection of malarial parasites
- Author Details:
-
Deepika *
-
Sathyavathi R Alva
Introduction
According to the latest World Malaria report, released on 30 November 2020, there were 229 million cases of malaria in 2019 compared to 228 million cases in 2018. The estimated number of malaria deaths stood at 409000 in 2019, compared with 411000 deaths in 2018.[1], [2], [3] Malaria is a serious disease caused by the protozoal parasite Plasmodium species, and if left untreated, can be fatal.[1] Due to the serious nature of P. falciparum infections, prompt and accurate diagnosis of the condition is essential for effective management. [4]
Keeping in mind the seriousness of the condition and the current availability of diagnostic facilities across India, we decided to compare the efficacy and accuracy of thick and thin peripheral blood smears [which is the gold standard for the diagnosis of malaria in endemic countries] with QBC (Quantitative Buffy Coat) by fluorescent microscopy and Antigen Card test for the detection of malarial parasites.
Materials and Methods
Inclusion criteria
Patients of all ages with clinical signs and symptoms of Malaria.
Exclusion criteria
Haemolysed or clotted sample.
Sample with microbial contamination.
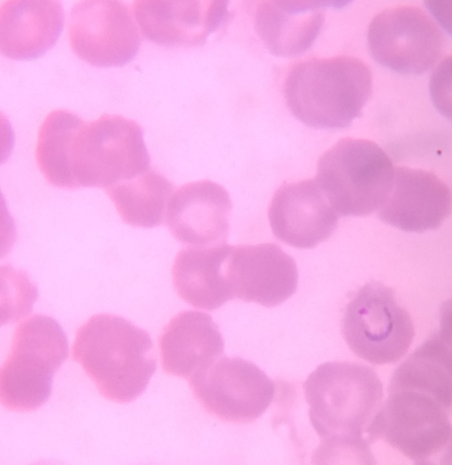
Thick and Thin Peripheral blood smear
These were prepared as per the standard method described elsewhere. The slides were examined by an experienced Pathologist/Microbiologist. Thick smears were reported negative after examination of 20-30 oil immersion fields when no parasites were observed; a thin smear was given negative when no parasites were observed in 20 oil immersion fields. [5], [6]
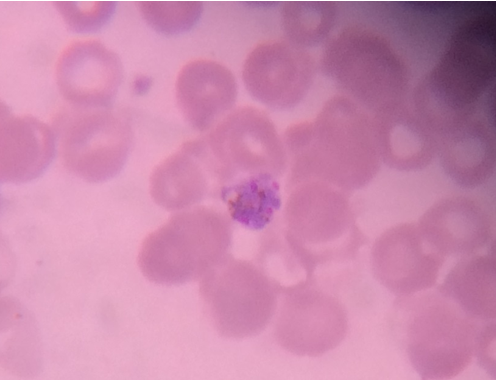
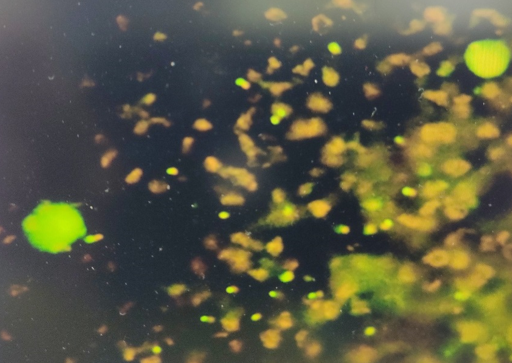
Quantitative buffy coat
Specially designed microhematocrit tubes coated with acridine orange were used. Approximately, 55-60 μl of blood was loaded into the tubes and stopper and float were applied at either ends; the tubes were centrifuged at 12000 RPM in a pre-programmed centrifuge as per the manufacturer’s instructions. . [5], [7] The interpretation was done using a standard microscope fitted with Para Lens ultraviolet microscope adaptor and a ×60 objective connected to fiber optic ultraviolet light module. The parasites were seen in buffy coat layer and the interface between RBC and WBC regions. Reports were given as +, ++, +++, ++++ for 0-1, 1-10, 10-100 and more than 100 parasites/QBC fields respectively.[5], [3], [8]
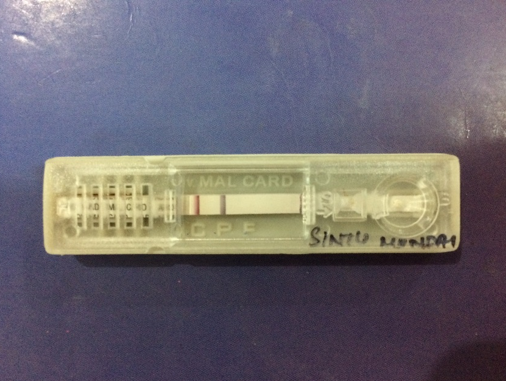
Antigen card test
Whole blood was collected in a clean container (containing EDTA). Commercially available antigen detection kit used. 4ul whole blood was taken using the sample dropper. The blood sample was added onto the sample pad in the sample well.3 drops of the assay buffer was added in the buffer well. The results were read after 20 minutes.[4], [9] Interpretation-Positive-Appearance of the three purplish pink colored lines one each in Pf. Region (F), Pan region (P) and Control region (C) indicated that the sample is reactive for P. falciparum and/or P.vivax/P.malariae/P.ovale only. Negative- Appearance of only one purplish pink coloured line at control ‘C’ region Invalid- if no line appeared.[4], [5]
Results
Out of 250 subjects, 150 were found to be positive by Thick peripheral blood smear. The rate of positivity and negativity was 60% and 40% respectively. In case of thin peripheral blood smear, 144 were positive and 106 were negative. The rate of positivity and negativity was 57.6% and 42.4% respectively. In Antigen Card Test, 155 were positive and 95 were negative with rate of positivity and negativity being 62% and 38%. In MPQBC, 162 subjects were found to be positive and 88 were negative. The rate of positivity and negativity was 64.8% and 35.2% respectively. ([Table 1])
Considering Thick PS as gold standard, the sensitivity, specificity and accuracy of Thin PS was 92.5%, 92.3% and 92.4% respectively. The sensitivity and specificity of Ag Card Test was 95.5%, 94.6% and 95.2%. The sensitivity, specificity and accuracy of MPQBC was found to be the highest among all i.e 98.7%, 97.7% and 98.4% respectively. The sensitivity and specificity of Thin PS was found to be the lowest.([Table 2])
|
Tests |
Results |
|
|
|
Positive |
Negative |
|
Thick Peripheral Smear (n = 250) |
150 (60) |
100 (40) |
|
Thin Peripheral Smear (n = 250) |
144 (57.6) |
106 (42.4) |
|
Antigen Card Test (n = 250) |
155 (62) |
95 (38) |
|
MPQBC (n = 250) |
162 (64.8) |
88 (35.2) |
|
Test |
Sensitivity (%) |
Specificity (%) |
Positive Predictive Value (PPV) (%) |
Negative Predictive Value (NPV) (%) |
Accuracy (%) |
|
Thin PS |
92.5 |
92.3 |
94.4 |
89.7 |
92.4 |
|
Ag Card Test |
95.5 |
94.6 |
96.8 |
92.6 |
95.2 |
|
MPQBC |
98.7 |
97.7 |
98.8 |
97.7 |
98.4 |
|
|
Thick PS (%) |
Thin PS(%) |
Ag Card Test (%) |
MPQBC (%) |
|
Our Study |
150 (60) |
144 (57.6) |
155 (62) |
162 (64.8) |
|
Panigrahi K |
62 (15.5) |
41 (10.25) |
76 (19) |
84 (21) |
|
Parija et al |
82 (19.95) |
45 (10.94) |
62 ( 15.09) |
66 (16.06) |
|
Gulrez et al |
49(61.25) |
49(61.25) |
71 (88.75) |
77(96.25) |
|
|
Thin PS |
Ag Card Test |
MPQBC |
|||
|
|
Sensitivity (%) |
Specificity (%) |
Sensitivity (%) |
Specificity (%) |
Sensitivity ( %) |
Specificity (%) |
|
Our Study |
92.5 |
92.3 |
95.5 |
94.6 |
98.7 |
97.7 |
|
Parija et al |
54.8 |
100 |
75 |
100 |
78.94 |
98 |
|
Panigrahi K |
66.12 |
100 |
93.0 |
94.67 |
96.7 |
92.89 |
Discussion
In many parts of the world, physicians often go with presumptive malaria diagnosis based on clinical symptoms and signs. Various clinical algorithms have been suggested and the best of them predict only up to 50% of true malaria cases. This method has poor specificity and positive predictive value. It does not allow differentiation of different species of malaria infection.[10], [11], [12]
In the present study, we found 150(60.00%) were positive by microscopic examination of Thick peripheral blood film, 144(57.6%) were positive by Thin PS, 155(62%) were positive by Ag Card Test and 162(64.8%) were positive by MPQBC respectively. The rate of positivity was highest with MPQBC method and lowest with Thin PS. The results were similar with a study conducted by Panigrahi et al,[10] where highest number of cases 84(21%) were positive by MPQBC method.([Table 3])
In a study by Parija et al, [5] they found 82(19.95%) cases were positive by Thick PS examination method which was the highest compared to the other methods, followed by MPQBC which detected 66(16.06%) cases. Examination of Thin PS has the least rate of positivity of 45(10.94%); the results were slightly different from our study, where the rate of positivity was highest with MPQBC method.([Table 3])
In another study done by Gulrez et al,[4] they found 77 (96.25%) cases were positive by MPQBC method, 49(61.25%), 48(69.25%) and 71(8.75%) were positive by Thick PS, Thin PS and Ag Card Test respectively. This was similar to our study where the rate of positivity was highest with MPQBC method and lowest by Thick PS examination.([Table 3])
In our study, taking Thick peripheral smear as gold standard, we found that thin smear had a sensitivity and specificity of 92.5% and 92.3% respectively. The sensitivity and specificity of Ag Card Test and MPQBC were 95.5%, 94.6% and 98.7%, 97.7% respectively.
In a study conducted by Parija et al [5] the sensitivity of Thin PS, Ag Card Test and MPQBC were 54.8%, 75% and 78.94% respectively. They found that sensitivity of MPQBC method was highest and Thin PS was the lowest which was similar to the results of our study. They found specificity of Thin PS and Ag Card Test to be 100% when compared to MPQBC method which was 98%. This result was different from our results where MPQBC method had the highest specificity i.e 97.7%.([Table 4])
In another study done by Panigrahi K,[10] the sensitivity of Thin PS, Ag Card Test and MPQBC were 66.12%, 93% and 96.7% respectively. They found the sensitivity of MPQBC method to be highest which was similar to our study. However, in their study, the specificity of Thin PS was found to be the highest and lowest for MPQBC method which was different from our study. The dissimilarities were may due to very small number of samples in this group than in our study.([Table 4])
Conclusion
MPQBC was found to be superior to other tests. It is the most accurate and efficient method for the detection of malarial parasites. Its simple, rapid, reliable, quick and easily mastered method for diagnosis of malaria. It is of much use in laboratories which screen large number of samples and in endemic areas where parasite level is low. It can also be used in highly suspected cases of malaria where parasite could not be detected in peripheral blood film.
In situations where adequate laboratory back up is not available, antigen card test can be employed. However, Leishman stained thin blood smear still appears superior for species identification.
Acknowledgement
Authors are thankful to Departments of Paediatrics and Medicine of K.V.G Medical College, Sullia D.K for providing institutional support to carry out this study.
Source of Funding
No financial support was received for the work within this manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- . World Health Organization. The “World malaria report 2019. 2019. [Google Scholar]
- C Wongsrichanalai, WH Wernsdorfer, S Muth, A Sutamihardja, MJ Barcus. A Review of Malaria Diagnostic Tools: Microscopy and Rapid Diagnostic Test (RDT). Am J Trop Med Hyg 2007. [Google Scholar] [Crossref]
- A Moody. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 2002. [Google Scholar]
- M Gulrez, K R Varshney, Y I Singh. Comparative study of various techniques in diagnosis of malaria. Sch J App Med Sci 2014. [Google Scholar]
- SC Parija, R Dhodapkar, S Elangovan, DR Chaya. A comparative study of blood smear, QBC and antigen detection for diagnosis of malaria. Indian J Pathol Microbiol 2009. [Google Scholar] [Crossref]
- P Berzosa, A de Lucio, M Romay-Barja, Z Herrador, V González, L García. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar J 2018. [Google Scholar] [Crossref]
- . World Health Organization. Regional office for South-East Asia. Malaria situation in SEAR countries. India. 2017. [Google Scholar]
- PL Bhandari, CV Raghuveer, A Rajeev, PD Bhandari. Comparative study of peripheral blood smear, quantitative buffy coat and modified centrifuged blood smear in malaria diagnosis. Indian J Pathol Microbiol 2008. [Google Scholar] [Crossref]
- MP Salmani, BM Preeti, BV Peerapur. Comparative study of peripheral blood smear and quantitative buffy coat in malaria diagnosis. J Commun Dis 2011. [Google Scholar]
- K Panigrahi. A Comparative study of peripheral blood smear, QBC and antigen detectiontest in diagnosis of malaria, in a tertiary care hospital. IJRRMS 2013. [Google Scholar]
- MJ Pinto, R Rodrigues, R Desouza, MP Verenkar. Usefulness of quantitative buffy coat blood parasite detection system in diagnosis of malaria. Indian J Med Microbio 2001. [Google Scholar]
- B. Sreekanth, S Shenoy, K Lella, N Girish, R Reddy. Evaluation of Blood Smears, Quantitative Buffy Coat and Rapid Diagnostic Tests in the Diagnosis of Malaria. J Bacteriol Parasitol 2011. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
Deepika , Alva SR. A Comparative study for the assessment of diagnostic efficacy and accuracy of various techniques in detection of malarial parasites [Internet]. IP J Diagn Pathol Oncol. 2025 [cited 2025 Sep 05];6(1):1-5. Available from: https://doi.org/10.18231/j.jdpo.2021.001
APA
Deepika, , Alva, S. R. (2025). A Comparative study for the assessment of diagnostic efficacy and accuracy of various techniques in detection of malarial parasites. IP J Diagn Pathol Oncol, 6(1), 1-5. https://doi.org/10.18231/j.jdpo.2021.001
MLA
Deepika, , Alva, Sathyavathi R. "A Comparative study for the assessment of diagnostic efficacy and accuracy of various techniques in detection of malarial parasites." IP J Diagn Pathol Oncol, vol. 6, no. 1, 2025, pp. 1-5. https://doi.org/10.18231/j.jdpo.2021.001
Chicago
Deepika, , Alva, S. R.. "A Comparative study for the assessment of diagnostic efficacy and accuracy of various techniques in detection of malarial parasites." IP J Diagn Pathol Oncol 6, no. 1 (2025): 1-5. https://doi.org/10.18231/j.jdpo.2021.001
