- Visibility 194 Views
- Downloads 15 Downloads
- DOI 10.18231/j.jdpo.2020.038
-
CrossMark
- Citation
Comparative evaluation of combined application of fine needle aspiration cytology and flow cytometry with histopathology for the diagnosis of Non-Hodgkin lymphoma
- Author Details:
-
Deepti Varshney *
-
Manju Kaushal
-
Minakshi Bhardwaj
-
Vijay Kumar
-
Palak Agarwal
Introduction
Non-Hodgkin lymphoma (NHL) is one of the commonest malignant neoplasms of the lymphoreticular system.[1] Lymphoma represents one of the major health problems all over the world and is one of the common cancer in the urban population in India, Delhi has the highest rates equivalent to 0.5 million cases per year.[2], [3] Fine needle aspiration cytology (FNAC) is commonly used in the investigation of lymphadenopathy and gives a rapid primary diagnosis. FNAC is less invasive and cost-effective.[4], [5] Unequivocal discrimination between low-grade NHL and reactive hyperplasia by microscopic observation is problematic in many cases; however, flow cytometry (FCM) due to its ability to measure multiple parameters on individual cells is ideal for the study of lymphoproliferative disorders on cytological material.[6] The combination of flow cytometric immunophenotyping (FCI) and FNAC have completely changed the approach to the cytological diagnostic algorithm of lymph node and lymphoid organs.[7] The possibility of applying a complete panel of antibodies and the diagnostic algorithm is the most appreciated advantage of this technique.[8] A combination of FNAC and FCM allows further sub-classification in most cases based on the new WHO classification. [9] FNAC and immunophenotyping by FCM are complementary and eliminate the need to perform an open biopsy for many patients with lymphadenopathy. [10], [11] Histopatological examination (HPE) is considered the gold standard method for the diagnosis and classification of lymphoma. However the need for anesthesia and possible complication of excisional biopsy limits its role, that’s why FNAC with FCM is gaining importance and has shown high sensitivity and specificity in the diagnosis of lymph node lesion, especially NHL. [12]
In our study, we evaluated and compared the efficacy of FNAC along with FCM with histopathology in the diagnosis and classification of NHL by using FCI on a sample obtained from the FNAC of lymph node tissue.
Materials and Methods
Study Population
Our study was a cross-sectional study, which was performed in the Pathology Department. This study was accepted by the Ethics Committee of our institute and written informed consent was taken from every patient.
FNAC was done to screen all patients of peripheral lymphadenopathy in all age groups. All Cases suggested as NHL were taken up for FCM as well as for excisional biopsy. Patients with non-neoplastic lesions of the lymph node and metastatic or Hodgkin’s lymphoma (HL) on FNAC were excluded from the study.
Methodology
FNAC and smear preparation
FNAC was done with a 23 gauge needle which was attached to a 10 ml syringe. The type of aspirated material obtained was noted. A thin and even film was made from the aspirated material on a clean glass slide. For Giemsa staining first air-dried smears were made and then fixed in methanol. For Papanicolaou staining smears were immediately fixed in alcohol and then staining was done.
Procedure for FCM
FCI of lymph node aspirate was performed by using four-color FCM (FACScalibur; Becton Dickinson). The instrument was calibrated each time a sample was run on the FCM. Antibodies bought from BD Biosciences; surface Ig kappa, surface Ig lambda, CD19, CD20 ,CD22, CD3, CD5, CD7, CD79b, FMC7, CD10, CD23, CD45,CD34 were used. CD19 or CD20 was used in all tubes and was used to identify the B cell population. A four-color analysis was performed using antibody bound to following fluorochrome FITC (Fluorescein isothiocyanate), PE (Phycoerythrin), PerCP (Perdinin chlorophyll protein), APC (Allophycocyanin).
Sample aspirated from fine-needle aspiration was collected in 1 ml sheath fluid in EDTA vial. For staining 10 µl of four antibodies, each conjugated to one fluorochrome was added to each tube. Tubes incubated in dark at room temperature for 30 minutes. Lysing- 2 ml of lysing solution (BD FACS lyse) in 1:10 dilution was added to each tube and incubated in dark at room temperature for 15 minutes. The centrifugation of tubes was done at 1200 rpm for five minutes, the supernatant was discarded and the pellet formed was broken by vortexing, then washing of the cells was done with 2 ml of sheath fluid by centrifugation. Cell suspension- the cells were then suspended in 0.5 ml of sheath fluid.
FCM data was acquired using the cell quest pro software. Instrument setting was done by opening the instrument setting files before acquiring the data. Each tube was run on an instrument one by one and events were acquired from each tube in the form of dot plots.
Steps for histopathology
The sections were processed in an automatic tissue processor (Shandon Citael 2000) at room temperature. A rotary microtome (Shandon) was used to cut the paraffin-embedded tissue blocks at 4µm thickness. Hematoxylin and eosin stain was used for staining the sections. Immunohistochemistry was performed on representative sections using the Avidin-Biotin technique. The appropriate precautions were taken during the handling of glassware, chemicals, and performing procedures to make results precise and accurate as well as diminish the personal threat.
Results
A total of 30 patients were taken in the study which includes 27 (90%) male and 3(10%) female patients. The maximum numbers of patients were noted in the age between 41-60 years ([Table 1]). A maximum number of patients presented with Generalised lymphadenopathy (46.7%) followed by cervical lymphadenopathy(40%) ([Table 2]). Nineteen (63%) patients presented with bilateral and eleven (37%) patients presented with unilateral lymph nodes. In all the cases FCM was done and subsequently analyzed. Out of the 30 cases, eight cases were not able to analyze because of the lack of events. In this study, we found that out of 22 cases, 18 cases were diagnosed as B-cell NHL, two cases as T-cell NHL, and two cases as reactive lymphoid hyperplasia. Thirteen out of 18 B-NHL (B-Non Hodgkin's lymphoma) cases presented with light chain restriction, eight cases showed Kappa while five showed Lambda restriction.
Subclassification was possible in 17 out of 22 cases on the basis of combined FNAC and FCM findings, three cases were diagnosed as B-NHL, and two cases as RLH ([Table 3]). The histopathology diagnosis was available in 29 out of 30 cases ([Table 4]). FCI with histopathology impression and cross-tabulation represented a total of 17 true positive cases, two true negative cases, seven false-negative cases, and no false-positive case. Whereas four cases could not be included because of two cases found to be inconclusive on histopathology due to crush and fixation artifacts, one case biopsy was not required as the case turned out to be common acute lymphoblastic leukemia-associated antigen-positive acute lymphoblastic leukemia (CALLA+ ALL) on hematological examination, and one case diagnosed as HL on histopathology while in this case, FCM was inconclusive due to paucity of events and on cytology differentials diagnosis of ALCL/HL (Anaplastic large cell lymphoma) was given ([Table 5], [Table 6]).
In our study 26 out of 30 cases were incorporated for predicting the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) which were found as 70.8%, 100%, 100%, and 22.2% respectively.




| Age range (Years) | Frequency (%) |
| ≤ 20 | 6(20) |
| 21-40 | 6(20) |
| 41-60 | 10(33.3) |
| >60 | 8(26.7) |
| Total | 30(100) |
| Lymph node | Frequency |
| Cervical | 12(40) |
| Axillary | 1(3.3) |
| Inguinal | 3(10) |
| Generalized | 14(46.7) |
| Total | 30(100) |
| FNAC FCI | Small cell type (4) | Small to Intermediate cell type(8) | Intermediate cell type (4) | Intermediate to large cell type (6) | Large cell type (6) | Polymorph -ous cells (2) |
| SLL | 4 | 2 | ||||
| MCL | 2 | |||||
| DLBCL | 2 | 4 | ||||
| B-LBL | 1 | |||||
| PTCL | 1 | 1 | ||||
| RLH | 2 | |||||
| B-NHL | 1 | 2 | ||||
| Not* analyzable | 2 | 2 | 2 | 2 |
| Histopathology | Number of cases (Total= 29) |
| SLL | 6 |
| DLBCL | 6 |
| MCL | 2 |
| FL | 2 |
| ALCL | 2 |
| PTCL | 1 |
| T cell histiocyte rich DLBCL | 1 |
| B-LBL | 1 |
| B-NHL | 2 |
| ALCL/HL | 1 |
| HL | 1 |
| RLH | 1 |
| Leukemic infiltration into LN | 1 |
| Inconclusive** | 2 |
| FCI+ FNAC | Histopathology | |||||||
| SLL | MCL | DLBCL | ALCL | PTCL | FL | Inconclusive** | Others | |
| SLL (6) | (6) | |||||||
| MCL (2) | (2) | |||||||
| DLBCL (6) | (6) | |||||||
| PTCL (2) | (1) | (1) | ||||||
| RLH (2) | RLH(1) B-NHL(1) | |||||||
| B-NHL (3) | (1) | (1) | B-NHL(1) | |||||
| Not Analysable* (8) | (2) | (1) | T cell/histiocyte rich DLBCL(1),ALCL/NSHL(1), B-LBL(1), HL(1), Leukemic infiltrate into LN(1) |
| FCM Diagnosis | Histopathology impression | Total | |
| Positive | Negative | ||
| Positive | 17 | 0 | 17 |
| Negative | 7 | 2 | 9 |
| Total | 24 | 2 | 26 |
Discussion
In this study maximum number of patients presented with generalized lymphadenopathy(46.7%) followed by cervical lymphadenopathy(40%). In the present study, the age range of patients was between 6 to 75 years which is comparable to the age range seen in other studies. Age range reported by Schmid et al. was 7 to 88 years and by Dey et al. was between 6 to 78 years. [6], [13] However, Barrena et al. reported the age range of 3 to 96 years in their study. [14]
On cytomorphology, the cases were classified as per morphological classification given by Orell showed a persistent number of small to intermediate, intermediate, intermediate to large, large, and polymorphous cell type.[15]
In our study, 22 out of 30 cases could be analyzed on FCM while 8 out of 30 cases could not be evaluated because of the paucity of events. Meda et al. also reported that inadequate material prevented proper FCM studies in 8.6% cases. [10] Failure to obtain adequate material was attributed to tumor necrosis, peripheral blood contamination, or insufficient aspirated material Dey et al. FCM analysis was done in 45 out of 48 cases while in 3 cases the material was insufficient because of poor blood mixed aspirate. [13]
In the present study, 22 cases were analyzed by FCI. Of these, 18 cases were diagnosed as B-NHL, two cases as T-NHL, and two cases as reactive lymphoid hyperplasia (RLH). B-NHL formed the major group and this was consistent with other studies. Dey et al. reported 40 out of 45 cases as B-NHL, Siebert et al. reported 36 out of 36 cases as B-NHL with no T-NHL, and Meda et al. reported 134 out of 158 cases as B-NHL and 6 cases as T-NHL. [10], [13], [16]
In our study, the light chain restriction could be established in 13 out of 18 cases of B-NHL (72%). It correlates very well with the study done by Dey et al. who could demonstrate light chain restriction in 75% cases, however Sayed et al. reported a light chain restriction in 100% of cases. [13], [17] This apparent lack of surface immunoglobulin is postulated to be due to the post-transcriptional defect of the immunoglobulin molecule.[18]
In this study, the majority of the cases which did not express light chain restriction were reported as SLL (Small lymphocytic lymphoma) (4/5 i.e. 80%) and one case was reported as DLBCL (Diffuse large B cell lymphoma). It correlates well with a study done by Pedro et al. who documented that out of 485 cases with specific lymphoma diagnosis, 46(9.5%) cases showed a discrepant light chain expression, and this discrepancy was seen maximum with the cases of SLL.[18]
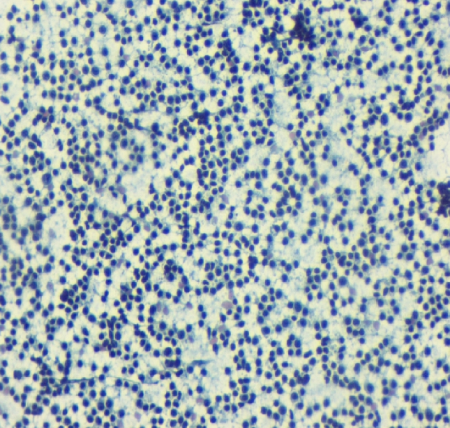
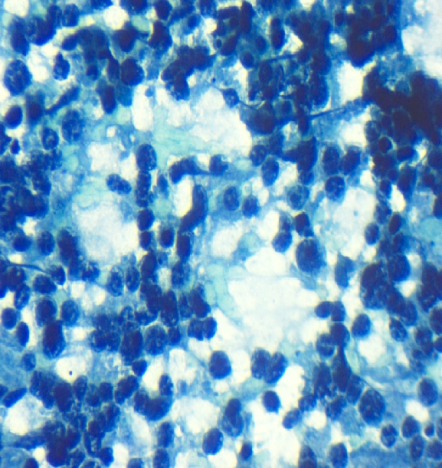
In our study by using combined FNAC and FCM, 17 out of 30 cases i.e. 56.6% were subclassified. Fifteen out of 18 cases of B-NHL and both cases of T-NHL were classified. Dey et al. subclassified 79% cases, Siebert et al. subclassified 76.3% of the cases while Meda et al. demonstrated subclassification in 90% of the cases. [10], [13], [16] The aforesaid variation in subclassification could be due to the lesser number of cases analyzed on FCM in the present study. In the subclassification, SLL cases showed light chain restriction, CD19 positive cell population along with an expression of CD5 and CD23 (Figure 1, Figure 2, and Figure 3). MCL cases showed light chain restriction, CD19 positive cell population along with CD5 positive, and CD23 negative expression. However, it was not possible to distinguish SLL and MCL cases by cytology alone. Individual cells on cytology smear show round to oval small nuclei and clumped chromatin with no nucleoli. DLBCL cases showed light chain restriction along with B cell marker positivity. On smear, DLBCL cases showed a monomorphic population comprising of intermediate to large cells or large cells showing coarse chromatin and prominent nucleoli (Figure 4, Figure 5, and Figure 6). There were four cases of SLL and one case of DLBCL which did not show light chain restriction but considering the cell morphology and predominant CD19 or CD20 expression, these cases were considered as B-NHL. This was in accordance to a study done by Dey et al. did not find light chain restriction in 10 out of the 40 cases of B-NHL and these cases showed predominant CD19 and CD20 cell population. [13]
On FCM of the two cases of T-NHL, one case of PTCL (Peripheral T cell lymphoma) showed CD5 expression, CD3 was heterogeneously expressed while CD7 showed dim positivity. The second case of PTCL showed positive expression of both CD5 and CD3 while CD7 was dim positive. Both of these cases showed negative expression of CD19. On cytology smear, these cases showed small to an intermediate and intermediate cell population with a round to oval nucleus, irregular nuclear membrane, coarse chromatin, inconspicuous nucleoli, and a moderate amount of cytoplasm. Based on cytomorphological features and T cell marker aberrancy these cases were diagnosed as PTCL. Similarly in the study done by Zeppa et al. reported the phenotype CD5+, CD19-, CD4+/CD8-, CD2-/CD3- and CD7+ or CD2+/CD3+ and CD7- in an appropriate cytomorphological setting was considered specific for the diagnosis of PTCL.[19]
In our study, DLBCL and SLL subtypes of NHL formed the majority comprising six cases each. Dey et al. found 17 out of 45 cases of DLBCL type, Sayed et al. reported 24 out of 45 cases of DLBCL type while Meda et al. reported 35 out of 158 cases as large B cell lymphoma which was maximum in, number in comparison to other types of lymphomas. [10], [13], [17] In the current study by using combined FNAC and FCM in the diagnosis of NHL when it was compared with HPE, sensitivity was found to be 70%, specificity 100%, Positive predictive value 100%, and Negative predictive value 22%. It was reported by Dey P et al. that the sensitivity of combined FNAC and FCI was 83.8% (26/31) and specificity was 100% (31/31) in the diagnosis of NHL however they also considered HPE as the gold standard. [13] Schmid et al. also noted a sensitivity of 98% and a specificity of 100% in their series. [6] Bangerter et al. showed that sensitivity of the combination of FNAC and FCM was 85.6%, specificity was 100%, the PPV was 100% and the NPV was 55.6%. [9] The low NPV in the present study could be due to small sample size and suboptimal sampling in 8 cases. Those 8 cases were included as false negative in statistical analysis.
In our study, two cases were diagnosed as RLH. On histopathological examination, one out of these two cases was diagnosed as RLH while discordance was noted in one case which was diagnosed as B-NHL and this case was taken as false negative in statistical analysis.
Three out of 22 cases analyzed on FCM could not be compared with histopathology one of these cases turned out to be CD10 positive acute lymphoblastic leukemia on hematological examination, therefore, not biopsied. In the remaining two cases results of histopathology were inconclusive due to marked crush and fixation artifacts.
In this study FCM could not be done in 8 cases because of a lack of events out of these, six cases were diagnosed as NHL on cytology while in two cases the differential diagnosis of anaplastic large cell lymphoma/Hodgkin lymphoma was given. These cases on histopathology turned out to be ALCL (two cases), FL (one case), T-cell histiocyte rich DLBCL (one case), ALCL/HL (one case), B-LBL (one case), HL (one case), leukemic infiltrate into LN (one case).
FCI in combination with cytomorphology can subclassify most types of B–NHL but for large cell lymphomas like ALCL and HL, FCI is not very helpful. Other upcoming techniques like cell proliferation marker and molecular markers may be helpful. Cytogenetics demonstrating chromosomal translocations may be required for definitive identification of the lymphomas.
Although for the diagnosis of NHL Biopsy and HPE is a gold standard; FNAC combined with FCI is a quick, less invasive, cost-effective, and reliable method that can be used as a first-line investigation in the diagnosis of NHL.
Acknowledgement
None.
Source of Funding
None.
Conflict of Interest
None.
References
- U N Saikia, P Dey, H Vohra, K G Subhash. DNA Flowcytometry of Non-Hodgkin’s Lymphomas: Correlation with Cytological grade and Clinical Relapse. Diagn Cytopathol 2000. [Google Scholar]
- E Naz, M Mirza, S Aziz, F Danish, S T Siddique, Ali A. Frequency and clinicopathological correlation of different types of NHL according to WHO classification. J Pak Med Assoc 2011. [Google Scholar]
- A Sharma, J Bajpai, V Raina, BK Mohanti. HIV-associated non-Hodgkin′s lymphoma: Experience from a regional cancer center. Indian J Cancer 2010. [Google Scholar]
- M. D. Jeffers, J. Milton, R. Herriot, M. McKean. Fine needle aspiration cytology in the investigation on non-Hodgkin's lymphoma. J Clin Pathol 1998. [Google Scholar]
- Uma Nahar Saikia, Pranab Dey, Biman Saikia, Ashim Das. Fine-needle aspiration biopsy in diagnosis of follicular lymphoma: Cytomorphologic and immunohistochemical analysis. Diagn Cytopathol 2002. [Google Scholar]
- S. Schmid, M. Tinguely, P. Cione, H. Moch, B. Bode. Flow cytometry as an accurate tool to complement fine needle aspiration cytology in the diagnosis of low grade malignant lymphomas. Cytopathol 2011. [Google Scholar]
- P Zeppa, E Vigliar, I Cozzolino, G Tronocone, M Picardi, A Renzo. fina needle aspiration cytology and flowcytometry immunophenotyping of Non Hodgkins lymphoma: can we do better?. Cytopathol 2010. [Google Scholar]
- Zahid Kaleem, Glenda White, Robin T. Vollmer. Critical Analysis and Diagnostic Usefulness of Limited Immunophenotyping of B-Cell Non-Hodgkin Lymphomas by Flow Cytometry. Am J Clin Pathol 2001. [Google Scholar]
- Markus Bangerter, Olaf Brudler, Bernhard Heinrich, Martin Griesshammer. Fine Needle Aspiration Cytology and Flow Cytometry in the Diagnosis and Subclassification of Non-Hodgkin’s Lymphoma Based on the World Health Organization Classification. Acta Cytologica 2007. [Google Scholar]
- B A Meda, D H Buss, R D Woodruff, J O Cappellari, R O Rainer, B L Powell. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol 2000. [Google Scholar]
- M Chosia, E Wolska-Szmidt. Is it possible to diagnose lymphoproliferative lesions by fine needle aspiration biopsy?. Klin Oczna 2005. [Google Scholar]
- F Ensani, S Mehravaran, G Irvanlou, M Aghaipoor, S Vaeli, E Hajati, Z Khorgami, S Nasiri. Fine-needle aspiration cytology and flow cytometric immunophenotyping in diagnosis and classification of non-Hodgkin lymphoma in comparison to histopathology. Diagn Cytopathol 2012. [Google Scholar]
- P Dey, T Amir, A Jassar, S Shemmar, S Jogai, G Bhat. Combined application of fine needle aspiration cytology and flowcytometric immunophenotyping for diagnosis and classification of Nonhodgkins lymphoma. Cytojournal 2006. [Google Scholar]
- S Barrena, J Almeida, Mdc Garcia-Macias, A Lopez, A Rasillo, J M Sayagues. Flowcytometry immunophenotying of fine needle aspiration specimens: utility in the diagnosis and classification of Non-Hodgkins lymphoma. Histopathol 2011. [Google Scholar]
- S R Orell, G F Sterrett. Orell and sterrett’s Fine Needle Aspiration Cytology. Lymph nodes 2012. [Google Scholar]
- J D Siebert, L M Weeks, L W List, J W Kugler, J A Knost, P A Fishkin. Utility of flow cytometry immunophenotyping for the diagnosis and classification of lymphoma in community hospital clinical needle aspiration/biopsies. Arch Pathol Lab Med 2000. [Google Scholar]
- A Sayed, M Borai, A Bahnassy, S Garzawi. Flowcytometric immunophenotyping of lymphoma:correlation with histopathology and immunohistochemistry. Diagn Pathol 2008. [Google Scholar]
- Pedro Horna, Horatiu Olteanu, Steven H. Kroft, Alexandra M. Harrington. Flow Cytometric Analysis of Surface Light Chain Expression Patterns in B-Cell Lymphomas Using Monoclonal and Polyclonal Antibodies. Am J Clin Pathol 2011. [Google Scholar]
- Pio Zeppa, Gilda Marino, Giancarlo Troncone, Franco Fulciniti, Amalia De Renzo, Marco Picardi. Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-hodgkin lymphoma. Cancer 2003. [Google Scholar]
How to Cite This Article
Vancouver
Varshney D, Kaushal M, Bhardwaj M, Kumar V, Agarwal P. Comparative evaluation of combined application of fine needle aspiration cytology and flow cytometry with histopathology for the diagnosis of Non-Hodgkin lymphoma [Internet]. IP J Diagn Pathol Oncol. 2025 [cited 2025 Sep 08];5(2):192-199. Available from: https://doi.org/10.18231/j.jdpo.2020.038
APA
Varshney, D., Kaushal, M., Bhardwaj, M., Kumar, V., Agarwal, P. (2025). Comparative evaluation of combined application of fine needle aspiration cytology and flow cytometry with histopathology for the diagnosis of Non-Hodgkin lymphoma. IP J Diagn Pathol Oncol, 5(2), 192-199. https://doi.org/10.18231/j.jdpo.2020.038
MLA
Varshney, Deepti, Kaushal, Manju, Bhardwaj, Minakshi, Kumar, Vijay, Agarwal, Palak. "Comparative evaluation of combined application of fine needle aspiration cytology and flow cytometry with histopathology for the diagnosis of Non-Hodgkin lymphoma." IP J Diagn Pathol Oncol, vol. 5, no. 2, 2025, pp. 192-199. https://doi.org/10.18231/j.jdpo.2020.038
Chicago
Varshney, D., Kaushal, M., Bhardwaj, M., Kumar, V., Agarwal, P.. "Comparative evaluation of combined application of fine needle aspiration cytology and flow cytometry with histopathology for the diagnosis of Non-Hodgkin lymphoma." IP J Diagn Pathol Oncol 5, no. 2 (2025): 192-199. https://doi.org/10.18231/j.jdpo.2020.038
