- Visibility 25 Views
- Downloads 6 Downloads
- DOI 10.18231/j.jdpo.2020.032
-
CrossMark
- Citation
Utility of FNAC in salivary gland swellings with clinico-histological correlation in a tertiary care hospital
- Author Details:
-
Prajna Das
-
Goutami DasNayak *
-
Neha Pandey
-
Kanaklata Dash
Introduction
Salivary gland lesions constitute about 2-6.5% of all head and neck lesions.[1] The annual incidence of overall salivary gland tumors worldwide is 0.4-13.5 cases per 100,000 populations. Incidence of malignant salivary gland tumors accounts for 0.4-2.6 per 100,000 populations worldwide.[2] FNAC is a rapid, reliable, safe, cost-effective and minimally invasive method for evaluating salivary gland lesions. It was first introduced in 1920 for assessment of parotid lesions and has gained popularity since then. Major salivary glands being superficial in location are easily accessible for FNAC. FNAC is the preliminary diagnostic tool which segregates non-neoplastic from neoplastic lesions and hence avoids unnecessary surgeries. However, due to heterogeneous and overlapping morphological features, cytological diagnosis of salivary lesions is most often challenging and not definitive. Hence, clinical, radiological and histopathological study in conjunction with cytology can help us to arrive at a definitive diagnosis.
Aims and Objectives
To study the cytomorphological spectrum of salivary gland lesions in our institution
To assess the diagnostic accuracy of FNAC by cyto-histopathological correlation
Materials and Methods
This is a prospective study over a period of two years conducted at the Department of Pathology, Kalinga Institute of Medical Sciences (KIMS), Bhubaneswar, Odisha from January 2018 to January 2020. 120 patients with palpable salivary gland lesions attending the Cytology section of our department were included in the study. A detailed clinical examination was done and radiological findings noted wherever available. Written informed consent of the patient or attendant was taken. Patients with non-palpable salivary lesions, bleeding tendencies and unwillingness for the procedure were excluded from the study.
FNAC was performed by using 22-G needle attached to a 10ml syringe holder by a free hand technique. A minimum of two needle passes were made in each case. The air-dried smears were stained with Leishman’s stain and wet ethanol fixed smears were stained by H&E. Special stains were used wherever needed. Some of the patients underwent surgery and our pre-operative cytological results were compared with histopathology wherever possible. Then the study was classified into four categories: True-negative (absence of malignancy correctly diagnosed), True-positive (presence of malignancy correctly diagnosed), False-negative (cytological specimen failed to diagnose a malignancy) and False-positive (cytological specimen was incorrectly considered or suspected for malignancy).
Observations and Results
Total number of 120 patients with palpable salivary gland swellings underwent FNAC in the Cytology section. Maximum number of patients (21.67%) belonged to age group of 41-50 yrs [[Table 1]] with a range of 11 to 80 yrs. Majority of cases were female 66 cases (55%)[[Table 2]]. Parotid swellings constituted about 58% of the total salivary gland swellings followed by swellings of submandibular gland (30%) and then the minor salivary glands comprising of 12% [[Table 3]].
On cytological examination, out of 120 cases, 57 cases (47.5%) were non-neoplastic lesions and 63 cases (52.5%) were neoplastic lesions [Table 4,5]. The most common non-neoplastic lesion was chronic Sialadenitis constituting 18 cases (31.5%) [[Table 4]]. The common benign lesion was Pleomorphic adenoma comprising of 33 cases (55.3%) [[Table 5]]. Among the neoplastic lesions, 43cases (68.2%) were benign and 20 cases (31.8%) were malignant [[Table 6]]. Among malignant lesions, the most common tumor was Mucoepidermoid carcinoma constituting 8 cases i.e 12.7% of all neoplastic lesions and 40% of all malignant lesions [[Table 5]].
Out of 120 patients, 62 cases underwent surgery. Their histopathological examination was done and correlated with cytological diagnosis. Out of 57 cases of non-neoplastic lesions on FNAC, 19 cases underwent surgery of which 16 cases were confirmed non-neoplastic, one case was benign tumor and 2 cases were diagnosed as malignant tumors by histopathology. Among 63 cytologically diagnosed neoplastic lesions, 43 cases were benign and 20 cases were malignant. Out of 43 benign neoplasms, 35 cases underwent biopsy and showed concordance with FNAC in 34 cases and discordance in 1case. Similarly out of the 20 malignant neoplasms diagnosed cytologically, only 8 patients underwent surgery; 7 cases turned out to be malignant and one case was discordant [[Table 6]].
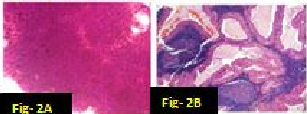
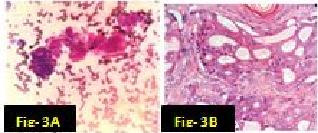
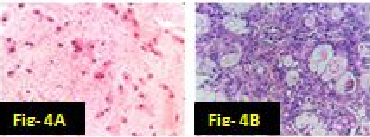
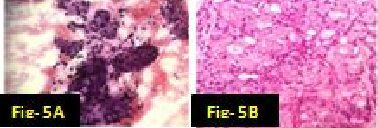
In our study there were many concordant and discordant results in cyto-histology correlation. 29 cases of Pleomorphic adenoma in cytology were confirmed by biopsy [[Figure 1] A,B]. . Out of 4 cases diagnosed by FNAC as suggestive of Warthin’s tumor, 3 cases were confirmed histologically [[Figure 2] A,B] and one was found to be salivary duct cyst on biopsy. 2 cases of Adenoid cystic carcinoma were proven by biopsy [[Figure 3] A,B]. One case diagnosed as mucus retention cyst in cytology was low-grade mucoepidermoid carcinoma histologically [[Figure 4] A,B]. One case which was diagnosed as sialadenosis cytologically came out to be Acinic cell carcinoma histologically [[Figure 5] A,B]. Out of 15 cases diagnosed as chronic sialadenitis, one was Warthin’s tumor, 13 were chronic sialadenitis and one was Kuttner’s tumor on histology. Two cases diagnosed as monomorphic adenoma cytologically were basal cell adenoma and oncocytoma on histopathology. Out of two cases of mucoepidermoid carcinoma in cytology, 3 cases were confirmed and one case was Pleomorphic adenoma in histology. One case of metastatic deposit with differential diagnosis of carcinoma-ex-pleomorphic adenoma was confirmed to be carcinoma-ex-pleomorphic adenoma on biopsy. Another case of metastatic deposit in submandibular gland came out to be metastatic squamous cell carcinoma [[Table 7]].
| Age group(yrs) | No. of cases | Percentage (%) |
| 11-20 | 08 | 6.7 |
| 21-30 | 17 | 14.2 |
| 31-40 | 20 | 16.7 |
| 41-50 | 26 | 21.7 |
| 51-60 | 19 | 15.8 |
| 61-70 | 14 | 11.7 |
| 71-80 | 16 | 13.3 |
| TOTAL | 120 | 100% |
| Gender distribution | No. of cases | Percentage (%) |
| Male | 54 | 45 |
| Female | 66 | 55 |
| Total | 120 | 100 |
| Site of FNAC | Number of cases | Percentage(%) |
| Parotid | 70 | 58 |
| Submandibular | 36 | 30 |
| Minor salivary glands | 14 | 12 |
| Cytological diagnosis | No. of cases | Percentage (%) |
| Benign salivary aspirate | 04 | 7.0 |
| Sialadenosis | 08 | 14.0 |
| Acute sialadenitis | 05 | 8.8 |
| Chronic sialadenitis | 18 | 31.5 |
| Granulomatous inflammation | 02 | 3.5 |
| Cystic lesion | 08 | 14.0 |
| Reactive hyperplasia | 05 | 8.8 |
| Abscess | 07 | 12.3 |
| Total | 57 | 100 |
| Cytological diagnosis | Number of cases | Percentage (%) |
| Pleomorphic adenoma | 33 | 52.3 |
| Warthin’s tumour | 08 | 12.7 |
| Monomorphic adenoma | 02 | 3.1 |
| Mucoepidermoid carcinoma | 08 | 12.7 |
| Adenoid cystic carcinoma | 05 | 7.9 |
| Metastatic carcinoma | 06 | 9.5 |
| Suggestive of Carcinoma ex-Pleomorphic adenoma | 01 | 1.6 |
| Total | 63 | 100 |
| Cases | No of FNAC cases | Histological follow-up cases | Non-neoplastic | Benign neoplasm | Malignant Neoplasm |
| Non-neoplastic | 57 | 19 | 16 | 01 | 02 |
| Benign | 43 | 35 | 01 | 34 | 0 |
| Malignant | 20 | 08 | 0 | 01 | 07 |
| Total | 120 | 62 | 17 | 36 | 09 |
| FNAC diagnosis(No of cases) | Histopathology diagnosis(No of cases) |
| Mucus retention cyst(1) | Low-grade Mucoepidermoid carcinoma(1) |
| Chronic sialadenitis(15) | Warthin’s tumour(1) Chronic sialadenitis(13) Kuttner’s tumour(1) |
| Sialadenosis(1) | Acinic cell carcinoma(1) |
| Reactive hyperplasia (1) | Reactive hyperplasia(1) |
| Abscess(1) | Infected salivary duct cyst(1) |
| Suggestive of Warthin’s tumour(4) | Salivary duct cyst(1) Warthin’s tumour(3) |
| Monomorphic adenoma(2) | Basal cell adenoma(1) Oncocytoma(1) |
| Pleomorphic adenoma(29) | Pleomorphic adenoma(29) |
| Mucoepidermoid carcinoma(4) | Pleomorphic adenoma(1) Mucoepidermoid carcinoma(3) |
| Adenoid cystic carcinoma(2) | Adenoid cystic carcinoma(2) |
| Suggestive of Metastatic deposit D/D- Carcinoma-ex Pleomorphic adenoma(1) | Carcinoma-ex-Pleomorphic adenoma(1) |
| Metastatic deposit in submandibular gland(1) | Metastatic squamous cell carcinoma(1) |
| FNAC diagnosis | H.P. diagnosis | Total | |
| Malignant | 7(TP) | 1(FP) | 08 |
| Non-malignant | 2(FN) | 52(TN) | 54 |
| Total | 9 | 53 | 62 |
| Studies | No of cases | Diagnostic accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Stramandinoli RT et al. | 79 | 82.3 | 68.2 | 87.7 | 68.2 | 87.7 |
| Piccioni et al | 176 | 97 | 81 | 99 | 93 | 98 |
| Stow N.et al | 104 | 92.3 | 86.9 | 92.3 | 96.8 | 86.6 |
| Postema RJ et al | 380 | 96 | 88 | 99 | 95 | 97 |
| Rehman H et al | 50 | 78 | 53.28 | 88.57 | 72.7 | 79.9 |
| Present study | 120 | 95.16 | 77.77 | 98.11 | 87.5 | 96.29 |

Statistical Analysis
The sensitivity, specificity, positive predictive value (PPV), negative predictive value(NPV) and overall diagnostic accuracy of FNAC to discriminate benign from malignant lesions were calculated by the following formulae of statistical analysis.
Sensitivity = True positive/ (True positive + False negative) x 100
Specificity = True negative/ (True negative + False positive) x100
Positive predictive value = True positive/ (True positive + False positive) x100
Negative predictive value = True negative/ (True negative + False negative) x100
Accuracy = True positive + True negative / (True positive + False positive + True negative + False negative) x100
In our study the sensitivity and specificity of FNAC was 77.77% and 98.11% respectively. The
Positive predictive value was 87.5% and Negative predictive value was 96.29% with a diagnostic accuracy of 95%.
Discussion
Our study included a total of 120 FNAC cases; of which 62 patients underwent surgery at our institution. Salivary gland lesions develop in a wide age range with maximum lesions belonging to age group of 41-50yrs (21.67%). In present study, benign tumours were prevalent between 31-40yrs and it correlated with studies by Ritu Jain et al [3] and Vidyadhara Rani et al.[4]
In the present study, 45 %( 54 cases) were males and 55 %(66 cases) were females. There was female preponderance with male to female ratio(M:F ratio) of 1:22. Our finding is comparable to the studies of N.Sangeetha et al[5] and Vidyadhara Rani et al.[4]
Parotid gland involvement was seen in 58%(70cases) of total FNAC cases which correlates with studies of Ritu Jain et al. [3] Shilpa H.Gandhi et al [6] and Kacharu et al.[7] Submandibular gland involvement was seen in 30%(36cases) and it is comparable with results in studies by Ritu Jain et al [3] and Sonal Verma et al.[8] The patients in our study presented with unilateral salivary gland swellings and there was no single case of bilaterality.
The present study showed non-neoplastic lesions to represent 47.5% of total cases and rest 52.5% were neoplastic lesions. These findings were comparable with studies by Hilda Fernandes et al.[9] The most common non-neoplastic lesion diagnosed was chronic siladenitis. Other non-neoplastic lesions were acute sialadenitis, sialadenosis, cystic lesion and abscess. These results showed consistency with studies by Mihashi et al and Ashraf et al.[10], [11]
Among benign neoplasms, Pleomorphic adenoma was the most frequently encountered lesion in our study which is well documented by Mihashi and Ashraf et al.[10], [11] Mucoepidermoid carcinoma was the most common tumor which was concordant with studies of Koirala S et al [12] and Panchal et al. [13]
On cyto-histopathological correlation, there were some discordant results. There were 7 true positive cases for malignancy which on biopsy comprised of Mucoepidermoid carcinoma (3cases), Adenoid cystic carcinoma (2cases), Carcinoma-ex Pleomorphic adenoma (1case) and Metastatic squamous cell carcinoma (1case). There was one false positive case of low-grade mucoepidermoid carcinoma on cytology which revealed pleomorphic adenoma on histopathology. The misdiagnosis in cytology was due to lack of typical features and presence of atypical looking squamous cells.
There were 52 true negative cases which revealed chronic sialadenitis(13cases), Kuttner’s tumour(1case), reactive hyperplasia(1case), infected salivary duct cyst(1case), salivary duct cyst(1 case), Pleomorphic adenoma (29cases), Warthin’s tumor (4cases), Oncocytoma (1case) and Basal cell adenoma(1case). Discordant result was seen in a parotid swelling diagnosed as suggestive of Warthin’s tumor due to presence of clusters of benign looking ductal cells, fluidy background, lymphocytes and cyst macrophages which turned out be just an infected salivary duct cyst on biopsy. On the contrary, 2 cases of chronic sialadenitis on FNAC revealed Kuttner’s tumor and Warthin’s tumour respectively on biopsy.
There were two false negative cases in our study. One case of Mucoepidermoid carcinoma was missed in cytology due to aspiration of only mucoid paucicellular fluid showing only muciphages in a mucoid background in FNAC. Similarly, a case of acinic cell carcinoma was misdiagnosed as sialadenosis as it showed clusters of normal looking acini.
The causes of non-diagnostic aspirate is most commonly due to sampling error which can be due to improper positioning of needle, cystic and haemorrhagic lesions.[14], [15] Representative, meticulous sampling and careful clinic-radiological correlation can help to reach a correct diagnosis. Small, non-palplable and deep seated lesions can be approached correctly by image guidance.
The sensitivity, specificity, positive predictive value and negative predictive values of FNAC in our study were 77.8%, 98.11%, 87.5 % and 96.3% respectively [[Table 9]] with a diagnostic accuracy of 95%, which was comparable with other studies.[16], [17], [18], [19], [20]
Conclusion
FNAC is an excellent preliminary diagnostic technique for planning the management of salivary gland lesions. However, it can be challenging due to overlapping features and heterogeneity in salivary gland lesions. Therefore FNAC should always be correlated with clinical, radiological findings along with histopathology to reach a definitive diagnosis.
Limitation of our study
The limitation of our study is less number of cases for biopsy due to loss to follow-up resulting in lack of histopathological correlation in all cases.
Source of Funding
The researchers did not receive any grant from outside funding agencies. This was self funding.
Ethical Approval
Prior to FNAC, all participants were oriented about the technique and informed consents were obtained.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- M M Khandekar, A N Kavatkar, B Khademi. FNAC of salivary gland lesions with histopathological correlation. Indian J Otolaryngeal Head Neck Surg 2006. [Google Scholar]
- Shivakumar Thiagarajan, Khuzema Fathehi, Deepa Nair, Anuja Deshmukh, Gouri Pantvaidya, DevendraAravind Chaukar, AnilKeith D'Cruz. Surgical morbidities and outcomes of major salivary gland neoplasms treated at a tertiary cancer center. Indian J Cancer 2018. [Google Scholar]
- R Jain, R Gupta, M Kudesia, S Singh. Fine needle aspiration cytology in diagnosis of salivary gland lesions:A study with histologic comparison. Cytojournal 2013. [Google Scholar]
- C A Rao, P V Rani. Diagnostic role of aspiration cytology in salivary gland lesions. J Chalmeda Anand Rao Ins Med Sci 2014. [Google Scholar]
- N Sangeetha, V Karthika, S Latha. Cytological analysis of salivary gland lesions with histopathological correlation. IJPBS 2013. [Google Scholar]
- Abhinav Singh, Bharathi M Purohit. Addressing geriatric oral health concerns through national oral health policy in India. Int J Health Policy Manag 2014. [Google Scholar]
- K T Dalve, S Y Swami, L U Rutuja, V V Narhire, A P Bakshi. Study of FNAC of salivary gland lesions in a tertiary care hospital. J Diagn Pathol Oncol 2016. [Google Scholar]
- Hemlata Verma, Rao S S, Verma V K. OBESITY IS AN UNAVOIDABLE ADVERSE DRUG REACTION TO ATYPICAL ANTIPSYCHOTICS. J Evol Med Dent Sci 2015. [Google Scholar]
- H Fernandes, D ’souza, C R Khosla, C George, L Katte, N H. Role of FNAC in the preoperative diagnosis of salivary gland lesions. JCDR 2014. [Google Scholar]
- H Mihashi, A Kawahara, M Kage, M Kojiro. Comparison of Preoperative Fine-Needle Aspiration Cytology Diagnosis and Histopathological Diagnosis of Salivary Gland Tumors. Kurume Med J 2006. [Google Scholar]
- Ameena Ashraf, Afsar Saeed Shaikh, Farrukh Kamal, Rahat Sarfraz, Mulazim Hussain Bukhari. Diagnostic reliability of FNAC for salivary gland swellings: A comparative study. Diagn Cytopathol 2009. [Google Scholar]
- S Koirala, G Sayami, AD Pant. Correlation of FNAC and histopathology in diagnosis of salivary gland lesions. J Pathol Nepal 2014. [Google Scholar]
- Panchal Upasana. A cytological and histological comparative study of salivary gland lesions at tertiary health care centre. Int Jf Biomed Adv Res 2015. [Google Scholar]
- S R Orell. Diagnostic difficulties in the interpretation of fine needle aspirates of salivary gland lesions: the problem revisited. Cytopathol 1998. [Google Scholar]
- J Klijanienko, A K El-Naggar, V Servois, J Rodriguez, P Validire, P Vielh. Mucoepidermoid carcinoma-ex pleomorphic adenoma : non-specidic pre-operative cytologic findings in six cases. Cancer 1998. [Google Scholar]
- RT Stramandinoli, LM Sassi, PA Pedruzzi, GH Ramos, BV Oliveira, DC Ogata, SO Ioshii. Accuracy, sensitivity and specificity of fine needle aspiration biopsy in salivary gland tumours: A retrospective study. Med Oral Patol Oral Cirugia Bucal 2009. [Google Scholar]
- L O Piccioni, B Fabiano, M Gemma, D Sarandria, M Bussi. Fine-needle aspiration cytology in the diagnosis of parotid lesions. Acta Otorhinolaryngol Ital 2011. [Google Scholar]
- Nicholas Stow, David Veivers, Alan Poole. Fine-needle Aspiration Cytology in the Management of Salivary Gland Tumors: An Australian Experience. Ear Nose Throat J 2004. [Google Scholar]
- Rolf J. Postema, Mari-Louise F. van Velthuysen, Michiel W. M. van den Brekel, Alfons J. M. Balm, Johannes L. Peterse. Accuracy of fine-needle aspiration cytology of salivary gland lesions in the netherlands cancer institute. Head & Neck 2004. [Google Scholar]
- H Rehman, M S Khan, F Wahid, I Ahmad. A profile of parotid gland tumours from a tertiary care hospital in Peshawar. J Postgrad Med Inst 2011. [Google Scholar]
