- Visibility 52 Views
- Downloads 16 Downloads
- DOI 10.18231/j.jdpo.2019.067
-
CrossMark
- Citation
Isolated primary endometrioid carcinoma of the ovary an unusual case of malignancy
- Author Details:
-
Kafil Akhtar *
-
Mohd Talha
-
Mohd Saquib
-
Shahid A Siddiqui
Introduction
Epithelial ovarian carcinoma remains the most lethal of gynecologic malignancies. Numerous studies have revealed the various histological subtypes of ovarian cancers might have distinguishing origination and mechanism of development, and divergent clinical and pathological characters and different prognosis as well.[1]
Ovarian endometrioid carcinoma (OEC) accounts for 16 – 25% of all epithelial ovarian cancers.[2] Compared with patients with high-grade serous carcinoma, a higher percentage of patients who are diagnosed with OEC are at the early stage, and the prognosis of this series of patients is relatively better. It is currently believed that patients with International Federation of Gynecology and Obstetrics (FIGO) stage I ovarian endometrioid carcinoma have a good overall prognosis and low rates of cancer recurrence.[3]
Most ovarian tumors are adenocarcinomas of different histological subtypes, derived from the surface epithelium of the ovary.[4] They manifest in various morphological forms as adenocarcinomas with serous, mucinous, clear cell or endometrioid differentiations. Also it is well known that primary endometrial neoplasms include the same subtypes.[5]
Case Summary
A 55-year-old woman presented with dull aching pain and lump in the right lower abdomen for the last 3 months. Ultrasonograpgy of the lower abdomen revealed a well defined mass in the right ovary. CT Scan confirmed the mass with hypechoic shadows in the right ovary with no noticeable ascitis. Hematogical and bichemical parameters were within normal limits, except a raised CA-125 levels of 154µg.
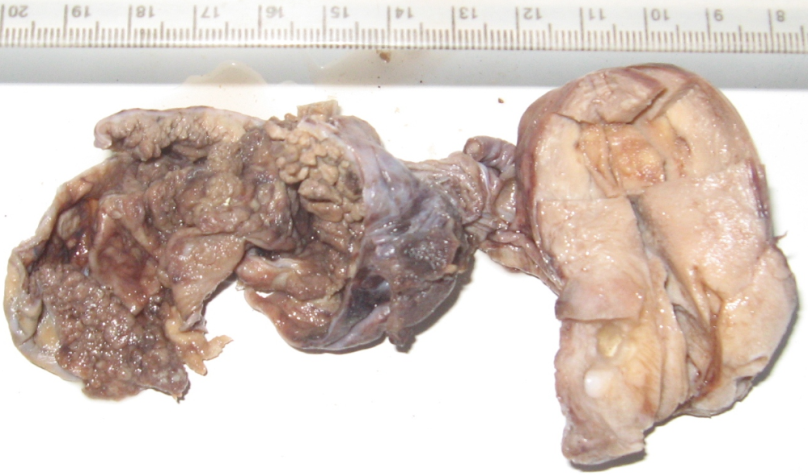
Hysterectomy with right oophorectomy was performed. Grossly, there was no evident intrauterine tumor with only foci of adenomyotic patches in the myometrium of the uterus and the endometrium was unremarkable with no involvement of the omentum. The right ovary was enlarged with smooth external surface, 10x 9.2 cms in size. Cut section showed solid to cystic growth with papillary configurations ([Figure 1]). There was no evidence of disease elsewhere in the abdomen with no lymphadenpathy, although there was a significant amount of fibrosis. The patient was staged as FIGO-IIA by Ovarian International Federation of Gynecology and Obstetrics classification.
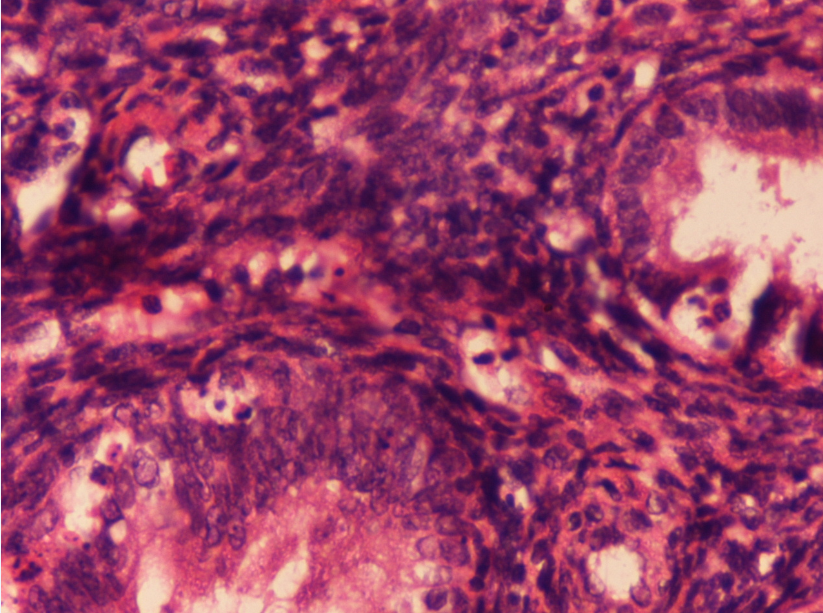
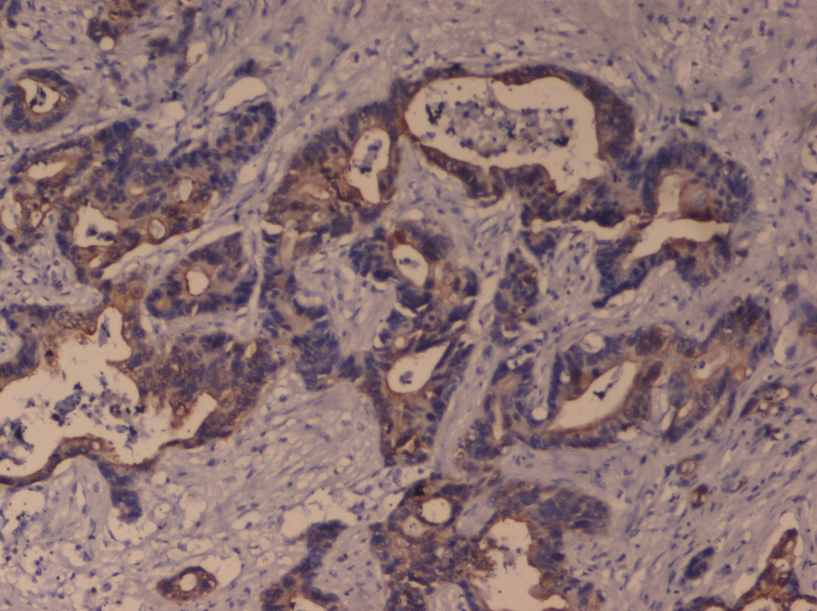
Microscopically, tissue sections showed closely packed glands lined by columnar epithelium with hyperchromatic basal nuclei, with minimal intervening stroma with foci of papillary configuration of tumor cells. Extensive areas of haemorrhage and necrosis was also noted with no lymphovascular invasion. ([Figure 2]). Immunohistochemical analysis showed the tumour cells reactive strongly with epithelial membrane antigen ([Figure 3]) and pancytokeratin and focally reactive with vimentin. A final diagnosis of endometrioid-type ovarian adenocarcinoma was given. Our patient was administered combination adjuvant chemotherapy of 6 cycles of 50 mg/m2 of carboplatin and 50 mg/m2 of paclitaxel.Our patient is doing well after 12 months of follow up.
Discussion
The mean age at diagnosis of all patients with ovarian cancer is 59.6 years.[2] Endometrioid carcinoma of the ovary is found to be more frequent in the younger age-group (<50 years), while mucinous and serous cancer occur a decade later.[3],[4] It has been suggested that endometrioid carcinomas have a better prognosis than do serous carcinomas, and present with lower histologic grade and FIGO staging.[4]
Some recent studies suggested that endometrioid adenocarcinoma admixed with serous, clear cell features may behave more aggressively than endometrioid adenocarcinoma without these features.[6],[7] Silva et al speculated that the endometrioid type EOC may become dedifferentiated into undifferentiated carcinoma in which the solid component of the tumour includes neoplastic cells with variable histological appearance, without developing glandular differentiation.[8]
EOCs are classified as serous, endometrioid, mucinous, clear cell, transitional, mixed and undifferentiated subtypes.[9],[10] The histological grade of EOC is decided by the cell architecture, nuclear pleomorphism and mitotic activity. Immunohistochemically EOCs react positively with epithelial membrane antigen (EMA), inhibin, pancytokeratin (panCK), and calretinin and in this way differ from sex-cord stromal tumours.[11] The endometrioid subtype reacts strongly with EMA and panCK as compared to the other types.[12],[13] Endometrioid adenocarcinomas developing in the endometrium and ovary most often stain strongly for vimentin, which greatly aids in distinguishing them from endometrioid or pseudoendometrioid tumours arising from the endocervix, colon or lung.[13] Our patient’s tumour cells strongly reacted with epithelial membrane antigen (EMA) and pancytokeratin (panCK), and focally reacted with vimentin, so we could clearly say that the tumour originated from the ovary.
Molecular markers emerging as mutation from PTEN and LOH analysis as described by Ricci et al may be more suitable to establish a correct final diagnosis in distinguishing between metastasis from primary synchronous carcinomas of the endometrioid subtype of the ovary and endometrium.[14] The potential of these molecular markers has to be evaluated in larger series, because so far this has been done in only few patients.[15],[16]
The 2016 National Comprehensive Cancer Network (NCCN) guidelines listed hormone therapy as a postoperative adjuvant treatment option for histologic grade 1 OEC and low- grade serous carcinoma; like aromatase inhibitors, leuprolide acetate, and tamoxifen.[17] But our patient of stage I OEC has responded well to platinum-based drugs and is doing well after 12 months of follow up period. Kumar et al in their study on 68 patients who received postoperative platinum- based combination chemotherapy have reported resistance to platinum-based chemotherapy drugs in only 3 patients (4.3%).[18] And there was no difference found for the DFS of patients with less than 4 cycles of platinum-based chemotherapy and more than 4 cycles.[19] It needs more efforts to investigate the optimal cycles of postoperative chemotherapy for Stage I OEC patients with variable prognostic risk factors.
The survival rate of patients with stage I OEC was higher than the survival rates of patients with stage I serous carcinoma and clear cell carcinoma.[19] In the present study, our patient was well after 12 months of follow up period.
Source of Funding
None.
Conflict of Interest
None.
References
- D J Dabbs, K Sturtz, R J Zaino. The immunohistochemical discrimination of endometrioid adenocarcinomas. Hum Pathol 2016. [Google Scholar]
- M Köbel, S E Kalloger, P M Baker. Diagnosis of ovarian carcinoma cell type is highly reproducible: a transcanadian study. Am J Surg Pathol 2014. [Google Scholar]
- R J Kurman, I M Shih. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 2012. [Google Scholar]
- M Köbel, S E Kalloger, D G Huntsman. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol 2010. [Google Scholar]
- D J Storey, R Rush, M Stewart, T Rye, A Al-Nafussi, A R Williams. Endometrioid epithelial ovarian cancer: 20 years of prospectively collected data from a single center. Cancer 2013. [Google Scholar]
- M Kobel, S E Kalloger, N Boyd. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 2018. [Google Scholar]
- C B Gilks. Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol 2014. [Google Scholar]
- S Silva, J Prat, M Stewart, T Rye, A Al-Nafussi, A R Williams. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol 2015. [Google Scholar]
- . Young RH and Scully RE. Metastatic tumors of the ovary, in Blaustein's Pathology of the Female Genital Tract, R. J. Kurman, Ed., Springer, New York, NY, USA, 2002;1063-1101. . . [Google Scholar]
- C C Niekerk, G P Vooijs, J Bulten, V A Dijck, A L Verbeek. Increased risk of concurrent primary malignancies in patients diagnosed with a primary malignant epithelial ovarian tumor. Mod Pathol 2017. [Google Scholar]
- W G Mccluggage, R H Young. Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol 2015. [Google Scholar]
- P Ramalingam, A Malpica, E G Silva, D M Gershenson, J L Liu, M T Deavers. The use of cytokeratin 7 and EMA in differentiating ovarian yolk sac tumors from endometrioid and clear cell carcinomas. Am J Surg Pathol 2014. [Google Scholar]
- M Kobel, J Bak, B I Bertelsen. Ovarian carcinoma histotype determination is highly reproducible, and is improved through the use of immunohistochemistry. Histopathol 2014. [Google Scholar]
- R Ricci, P Komminoth, F Bannwart, J Torhorst, E Wight, P U Heitz. PTEN as a molecular marker to distinguish metastatic from primary synchronous endometrioid carcinomas of the ovary and uterus. Diagn Mol Pathol 2013. [Google Scholar]
- J D Seidman, P R Rusell, R J Kurman. Surface epithelial tumors of the ovary,” in Blaustein's Pathology of the Female Genital Tract, R. J. Kurman, Ed., , Springer, New York, USA, 2012;791-904. . . [Google Scholar]
- R J Morgan, D K Armstrong, R D Alvarez, J N Bakkum-Gamez, K Behbakht, L M Chen. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 2016. [Google Scholar]
- W Sieh, M Kbel, T A Longacre, D D Bowtell, A De Fazio. Hormone receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol 2013. [Google Scholar]
- A Kumar, N Le, A V Tinker, J L Santos, C Parsons, P J Hoskins. Early-Stage Endometrioid Ovarian Carcinoma Population-Based Outcomes in British Columbia. Int J Gynecol Cancer 2014. [Google Scholar]
- F Vernooij, A P Heintz, P O Witteveen, M V Heiden, K W Coebergh, Y Van Der Graaf. Specialized care and survival of ovarian cancer patients in The Netherlands: nationwide cohort study. J Nat Cancer Inst 2008. [Google Scholar]
