Introduction
Hepatoid adenocarcinoma (HAC) is a very rare malignant tumor of extrahepatic origin which is characterized by having foci with features of both hepatocellular differentiation as well as adenomatous differentiation. It is most commonly seen in the stomach, however, few cases of HAC originating in the ovary, small intestine, colon, lung, uterus, pancreas, gallbladder, oesophagus and periampullary region have also been reported.1,2,3 Interestingly, it morphologically and functionally resembles Hepatocellular carcinoma, which can make the diagnosis challenging. Identification of HAC is necessary as it is associated with a dismal prognosis. We report here a case of a metastatic hepatoid adenocarcinoma of an unknown primary presenting in the ascending colon with both hepatoid and mucinous adenocarcinomatous areas on morphology.
Case Report
A 55 year old male patient presented to the surgery O.P.D. of our institute with progressive weakness of body accompanied by weight loss for three months and few episodes of haematochezia in the past fifteen days. He was advised testing for CBC, LFT and KFT, all of which were within normal limits. An ultrasonography of abdomen showed a large circumferential growth in mid part of ascending colon, near the hepatic flexure. Following this the patient was advised for CEA and CA19-9 levels which were within normal limits. The CT scan confirmed the USG findings and additionally revealed numerous necrotic mesenteric lymph nodes as well as necrotic areas in the nearby duodenum (? tumor infiltration). Rest all visceral organs were unremarkable. The patient underwent colectomy and partial duodenectomy. The specimen was received in two vials. In first vial a resected colonic segment was received measuring 18cm. in length, in the centre of which a large greyish white friable tumor mass with few haemorrhagic areas was seen measuring 12x8x5 cm. [Figure 1] On dissecting the attached mesentry, five lymph nodes were identified, largest measuring 1x1 cm. in size. In Vial II, a part of duodenum was received measuring 10 cm. in length, on the outer surface of which a necrotic area was seen measuring 7x2x 4 cm. [Figure 2] The mucosal surface was unremarkable. Sections from various representative areas of both the specimens were taken and processed.
Sections examined from the colonic tissue showed abundant areas of necrosis along with highly pleomorphic tumor cells that were large with round central nuclei,vesicular chromatin, prominent nucleoli and moderate amount of eosinophilic to clear cytoplasm. Predominantly se en in diffuse sheets, at some places these tumor cells were arranged in cords/rows. Mitotic activity was high.[Figure 3] One section showed admixture of these areas with mucinous adenocarcinoma with lot of signet cells.[Figure 4] All the five lymph nodes dissected from the attached mesentry showed tumor invasion. Sections examined from the duodenum showed normal mucosal lining with diffuse infiltration of the muscle layer and serosa by tumor cells of similar morphology as seen in the colon.[Figure 5] Numerous tumor emboli were also seen.[Figure 6] Based on these findings, a possibility of hepatoid adenocarcinoma of the colon was suggested and se rum AFP level estimation was advised. However, the patient had expired by then, so it could not be done. IHC for CK 7, CK 18, CK 20 and Hep- Par 1 was done to confirm our diagnosis. The tumor was strongly positive for CK 18 [Figure 7] and focally positive for Hep – Par 1 [Figure 8], confirming our diagnosis of Hepatoid Adenocarcinoma but to our surprise, it was negative for CK 20 [Figure 9] and positive for CK 7 [Figure 10], which implied that this was not of a intestinal origin and the primary was somewhere else. However, since the patient had expired, the primary origin could not be assessed. A possibility of lung origin was thought of on the basis of IHC finding of CK 7+ an d CK20-, since all the other abdominopelvic organs were reported to be completely unremarkable on CT scan.
Figure 3
Pleomorphic large tumor cells in sheets with large central nuclei, prominent nucleoli and moderate amount of eosinophilic to clear cytoplasm[H and E X 10x]
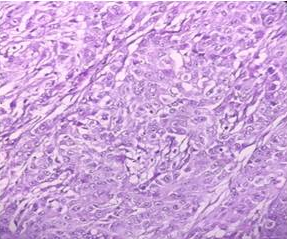
Figure 5
Sheets of tumor cells invading the duodenal wall with similar morphology as seen in the colonic tumor [H and E X 4x]
Discussion
HAC is a rare extrahepatic neoplasm that was first described by Bourreille and colleagues in 1970. It was termed as Hepatoid Adenocarcinoma by Ishikura and colleagues in 1985 due to the high AFP levels, ranging from 4730 to 700 000 ng/mL seen in serum of these patients.4,5,6 Kodama et al described two histologic types of HAC: one was the medullary type, characterized by polygonal cells arranged in a solid nests or sheets, with scattered large pleomorphic or multinucleated giant cells, and the other was well-differentiated papillary or tubular type with clear cytoplasm, sometimes both patterns coexisting in a single tumor.7
HACs are most commonly seen in the stomach, however, few cases of HACs originating in the ovary, small intestine, colon, lung, uterus, pancreas, gallbladder, oesophagus and periampullary region have also been reported.1,2,3
HAC occurs more often in males, with a male-to-female ratio of 2.3:1. The median age at occurrence is 63 ± 12.8 years. The majority of patients especially in the colorectal cases, present with haematochezia. Our patient too was an elderly male with similar presenting features. Most cases of HACs have increased serum AFP, with extremely high levels (> 1000 ng /mL), but few AFP-ve HACs have also been diagnosed. An absence of AFP overproduction does not exclude a diagnosis of HAC. Also the capacity of tumor tissue to produce AFP has no impact on prognosis. Notably, other serological biomarkers such as CEA and CA 19-9 are generally within normal ranges.6,8 In this case too, these tumor markers were within normal limits. However, AFP levels in our case could not be assessed as the patient had expired.
Fei et al in their study observed that AFP producing Colorectal cancers arise mainly from the ascending (40%) and sigmoid (35%) colon and the majority of patients have poor differentiation (50%), advanced local invasion (80%), lymph node (LN) metastasis (60%) with synchronous distant metastasis commonly present(35%).9
The cause and molecular mechanism of onco genesis of hepatoid adenocarcinoma in the colon are not clear. A case of hepatoid adeno carcinoma in the small intestine with Crohn’s disease has been reported. Hepatoid adenocarcinomas in the Barrett’s esophagus have also been seen, which indicates a possible association between them. To date, mo st tumors reported in the large bowel and all those reported in the small bowel have occurred along with inflammatory bowel diseases which strongly indicates a correlation between these two. Some studies have detected Her2 amplification and p53 mutations in gas tric HACs.10
Well to moderately differentiated HACs should be distinguished from metastatic Hepato Cellular Carcinomas [HCCs] as in both the arrangement of tumor cells is in a trabecular pattern along with hyaline globules and bille pigment commonly present. 6,11 Poorly differentiated HACs should be distinguished from poorly differentiated adenocarcinomas as both of them show sheets and cords of large, polygonal, eosinophilic tumor cells.12 So the diagnosis of HAC relies heavily on immunohistochemistry (IHC).
As an AFP producing tumor, HAC commonly [> 90%] stains for AFP regardless of organ origin. AFP is usually secrete d by HCC and gonadal tumors too, so AFP positivity is seen in tissues of these tumors. Apart from AFP, major IHC markers used for HACs include Glypican 3, CEA, CK18, and CK19. Glypican-3 is positive in almost all HACs. The proportion of HACs with a positive CEA stain is high in both gastric (75%) and non-gastric HAC (75%-100%), but it can also be positive in HCC and other metastatic adenocarcinomas arising from the gastrointestinal tract. Among epithelial markers, AE1/AE3, CK18 and CK19 are usually positive for HACs (92.3%, 100% and 100%) respectively.13
Hep Par 1 staining is usually negative in HAC, but it may occasionally show positive staining depending on the organ of origin, being positive in 100% of pancreatic, gallbladder and urinary gallbladder HACs but only in 31% of gastric HACs.1 Moreover, apart from HCCs, other tumors such as adrenal cortical carcinomas, yolk sac tumors, ovarian carcinomas may be occasionally positive for Hep Par 1 stain.13 Other stains for HACs such as α1- antitrypsin, α1-antichymotrypsin, CD10, CDX2, MUC1, PLUNC, SALL4 have been reported to be quite promising. 12
In our case too, CK 18 and Hep-Par 1 was positive. Because of CK 7 positivity and CK 20 negativity, a primary of the GIT was ruled out. The primary sites thought of were pulmonary/ pancreatobiliary. Unfortunately, since the patient had expired, the primary site could not be determined.
HACs have a dismal prognosis with high incidence of distant metastasis, especially to liver and lymphoid tissue and high mortality even after multimodality treatment.14 Because HAC mimics HCC, there may be a benefit from using therapies for HCC in HAC. Improved understanding of the biology of HAC may allow for recognition of therapeutic targets and development of improved treatment options for this aggressive disease.15
Conclusion
Though Hepatoid Adenocarcinomas are rare tumors of the GIT, awareness about them is very important because of their poorer prognosis compared to conventional adenocarcinomas. They can range in differentiation from well differentiated forms where they closely mimic Hepatic cell carcinomas to poorly differentiated forms where they closely mimic Poorly differentiated adenocarcinomas, moreso because of their frequent admixture with mucinous adenocarcinomatous areas. A high index of suspicion and use of appropriate IHC markers, especially in the setting of high AFP levels may clinch the diagnosis, though its important to remember that AFP negative ones also exist.








