Introduction
Salivary gland neoplasms represent <1% of all tumors and 3–5% of all head and neck neoplasms.1 There are between 450 and 750 minor salivary glands in the head and neck region, scattered throughout the sinonasal cavities, oropharynx, larynx and trachea with the majority being found in the oral cavity.2 80% of the tumors are benign in major salivary glands whereas 80% or more of minor salivary gland tumors are malignant2,3 and they tend to have a great variation in presentation and histology.2,4
The minor salivary gland tumors (MSGTs) can appear at any age; the maximum incidence is in the fourth decade of life for benign lesions and in the sixth decade for malignant tumors.5 The etiopathogenesis of MSGTs remains unclear. MSGTs have a high recurrence rate (5-30%) when surgical removal is incomplete, and the possibility of malignant transformation must be taken into consideration.1 This capacity to relapse is related to the histopathological characteristics of the tumor, and particularly to the initial treatment provided.
The present study describes seven cases of MSGTs. This includes pleomorphic adenoma, one in nasal cavity and two in parapharyngeal space and adenoid cystic carcinomas, three in nasal cavity and one in bronchus with varied presentations, revealing benign and malignant characteristics of the same.
Materials and Methods
This is a prospective analysis of the MSGTs conducted from January 2018 to June 2019 in our tertiary care center. The study comprised of seven patients. All patients presented with diverse clinical features in relation to their tumor location such as nasal obstruction, epistaxis, dysphagia, neck swelling. Among these cases, one patient presented with a unique clinical feature of unconsciousness to the emergency and was diagnosed as a case of cerebrovascular accident (CVA) which on further examination revealed a parapharyngeal mass. All the patients were investigated in the form of complete hemogram and computed tomography (CT scan).
Patients have undergone surgical excision of the tumor masses and was sent to our department of pathology. Gross examination of the specimens was done and sections were taken from the representative areas of the tumor masses. Different sections were embedded in paraffin, and cut and stained in the manner of histologic sections. The sections were reported by using light microscope at different magnification. The specimens were subjected to immunohistochemistry (IHC) by peroxidase-antiperoxidase technique. Epithelial membrane antigen (EMA) [ Monoclonal Mouse Antibody; clone: E29], S-100 [Polyclonal Rabbit Antibody; clone: 15E2E2], cytokeratin 7 (CK 7) [Monoclonal Mouse Antibody; clone: OV-TL12/30], vimentin [Monoclonal Mouse Antibody; clone: V9], p63 [Monoclonal Mouse Antibody; clone: 4A4] were being used for the confirmation of the diagnosis.
Results
Case 1
(Pleomorphic adenoma of the nasal septum)
A 10-year-old girl presented to the ENT OPD with nasal obstruction for last 6 months. It was non-tender, firm, polypoid mass covered by mucosa arising from the right nasal septum. Radiological examination (CT scan) showed a polypoidal mass in the anterior aspect of right nasal cavity, arising from the septum. Endoscopic removal of the mass was done. The specimen showed a well circumscribed globular greyish soft tissue mass measuring 2 × 2 × 1.5 cm (Figure 1 A). Histological examination showed a tumor mass composed of myxoid stroma with scattered glandular epithelial structures resulting in a melting pattern (Figure 2 B, C, D).
Figure 1
Pleomorphic adenoma of nasal septum (A) Gross specimen (B) Section shows salivary gland acini (indicated by arrow) with tumor mass (hematoxylin and eosin staining, x100, H&E) (C) Section shows chondromyxoid stroma (hematoxylin and eosin staining, x400, H&E) (D) Section shows melting pattern (hematoxylin and eosin staining, x 400, H&E)

It was diagnosed as pleomorphic adenoma. On IHC, EMA (Epithelial membrane antigen) and CK 7 stained positive for the ductal cells. S-100 and p63 stained positive for the myoepithelial cells. This confirmed our diagnosis pleomorphic adenoma of the nasal septum.
Case 2
(Adenoid cystic carcinoma of the nasal cavity)
A 41-year-old female presented to ENT OPD with right nasal obstruction from last 4 years and epistaxis from the same side for 3 months. On anterior rhinoscopy, mass was seen in right nasal cavity occupying the floor and the lateral wall of the nose displacing the septum to left. CT PNS revealed soft tissue attenuation filling the right maxillary, ethmoid, frontal, and sphenoid sinuses, which is filling the right nasal cavity and extending into right side of nasopharynx (Figure 2A). The tumor mass was removed by right lateral rhinotomy approach. Grossly, the mass was measuring 3 × 2×1.5 cm, greyish white, fleshy & soft to firm in consistency. On microscopy, It revealed respiratory epithelial lining with tumor mass underneath having a cribriform like pattern (Figure 2D). Tumor cells were small basaloid type with little pleomorphism.
Figure 2
Adenoid cystic carcinoma of the nasal cavity (A) CT scan showing soft tissue mass (B) IHC staining for p63 showing positivity (x400) (C) IHC staining for EMA showing positivity (x400) (D) Section shows the cribriform pattern (hematoxylin and eosin staining, x100, H&E)
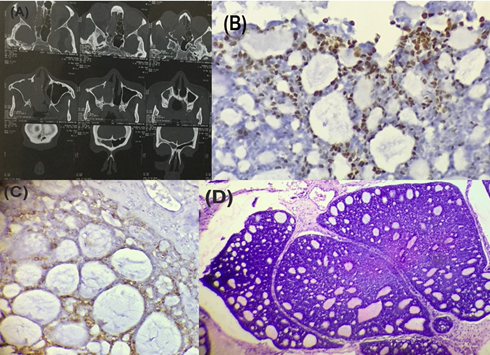
The final diagnosis was adenoid cystic carcinoma (Grade 2) of the nasal cavity. Immunohistochemical staining for p63 (Figure 2B), vimentin showed positivity in myoepithelial cells and EMA positivity (Figure 2C) for the ductal cells which confirmed our diagnosis.
Case 3
(Adenoid cystic carcinoma of the nasal septum)
A 54-years-old male patient presented to ENT OPD with left nasal swelling which started one year before and gradually increasing in size. Associated symptoms were dull aching pain for last 6 months and occasional epistaxis for past 15 days. On anterior rhinoscopy, a pinkish mass was seen obliterating the left nasal cavity adjacent to the septum. CT showed a soft tissue density lesion seen in left nasal cavity attached to nasal septum causing the widening of the left nasal cavity. The tumor along with septum was excised. Grossly, the mass measured 2.5 × 2 × 1 cm (Figure 3 A). The histological section showed a tumor mass composed of nests of cells arranged in cribriform pattern and gland like spaces filled with eosinophilic material (Figure 3 D).
Figure 3
Adenoid cystic carcinoma of the nasal septum (A) Gross specimen (B) IHC staining for vimentin showing positivity (x400) (C) IHC staining for CK 7 showing positivity (x400) (D) Section shows the cribriform pattern gland like spaces filled with eosinophilic material (hematoxylin and eosin staining, x100, H&E)
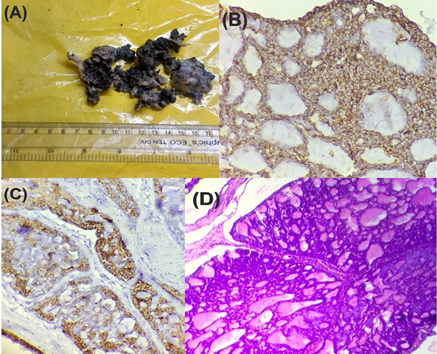
Individual cells were basaloid with scanty cytoplasm, oval nuclei, inconspicuous nucleoli. The overall features were in keeping with adenoid cystic carcinoma (Grade 2). Myoepithelial cells stained positive for p63, vimentin (Figure 3 B) and ductal cells stained positive for cytokeratin 7 (CK 7) (Figure 3 C) and this confirmed our diagnosis.
Case 4
(Pleomorphic adenoma of the parapharyngeal space)
A 35-year-old female presented to ENT OPD with a painless, slow growing right neck mass for 2 years which was increasing in size for last 2 months with a complaint of dysphagia. On intraoral examination, there was a firm bulging of the right soft palate and right lateral pharyngeal wall. The patient underwent CT scan which showed a large well-defined SOL in the right parapharyngeal region with a mass effect compressing the nasopharynx and oropharynx and displacing the pterygoid plate and pterygoid muscles (Figure 4A). Surgical excision of the lesion was done. Grossly, it was globular tissue piece measuring 6 × 4 × 3 cm (Figure 4B) and on cut open white slimy myxoid areas were noted. Histologically, the section showed a tumor mass having tubular glands lined by epithelial and myoepithelial cells and glands are seen to be embedded within the chondromyxoid stroma (Figure 4C).
Figure 4
Pleomorphic adenoma of the parapharyngeal space (A) CT scan shows large well-defined SOL (B) Gross specimen (C) Section shows glands embedded within the chondromyxoid stroma (hematoxylin and eosin staining, x100, H&E) (D) IHC staining for S100 showing positivity (x400)
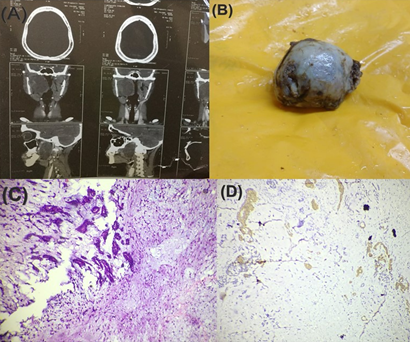
The final diagnosis of pleomorphic adenoma of the parapharyngeal space was made. EMA (Epithelial membrane antigen) and CK 7 stained positive for the ductal cells. S-100 (Figure 4D) and p 63 stained positive for the myoepithelial cells. Thus, confirmed our diagnosis.
Case 5
(Adenoid cystic carcinoma of the bronchus)
A 32-year-old male presented with dyspnea as well as dry cough for 1 year. For last six months he complaint of hemoptysis. There was no history of contact with pulmonary tuberculosis or past treatment for it. On chest auscultation, crepitations and rhonchi were present on right basal region. Spirometry suggested an obstructive abnormality. X-ray chest revealed collapse consolidation on right side (Figure 5 A, B). CT scan of chest revealed mass lesion in right bronchus with its complete obstruction and collapse consolidation of right lung. Left side of lung parenchyma appeared to be normal. Fiberoptic bronchoscopy showed a lobulated growth with sharp margins completely obstructing the right main bronchus. Complete excision of tumor was done. Histopathological examination showed normal respiratory lining with underlying tumor arranged in predominantly in cribriform pattern along with tubular pattern with many clusters showing central myxoid matrix (Figure 5C).
The tumor cells were compact, polyhedral with a round hyperchromatic nucleus and moderate amount of cytoplasm and was diagnosed as adenoid cystic carcinoma. Vimentin (Figure 5D) stained positive for myoepithelial cells and CK7 & EMA stained positive for ductal cells. The final histopathological diagnosis was adenoid cystic carcinoma (Grade 2) of bronchus.
Figure 5
Adenoid cystic carcinoma of the bronchus (A) Chest X-ray (AP view) revealed collapse consolidation on right side (B) Corresponding Chest X-ray (lateral view) (C) Section shows the cribriform pattern (hematoxylin and eosin staining, x100, H&E) (D) IHC staining for vimentin showing positivity (x400)
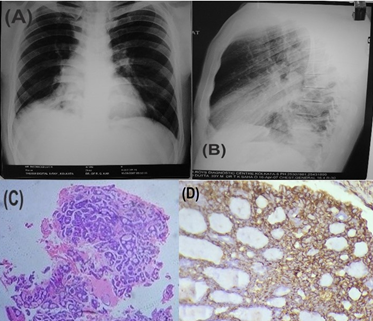
Case 6
(Adenoid cystic carcinoma of the nasal cavity)
A 40-year-old male presented with right nasal obstruction which was gradually progressing for last 2 years. There were no other significant comorbidities. Anterior rhinoscopy showed a mass in the right nasal cavity. The CT scan of paranasal sinus showed mass in right nasal cavity eroding anterior part of medial wall of maxillary sinus (Figure 6 A). The tumor mass was removed through right rhinotomy approach. The specimen measured 2 × 2 × 1 cm (Figure 6B). On microscopic examination, the section showed a tumor mass arranged in cribriform pattern with basaloid type of tumor cells (Figure 6C).
Figure 6
Adenoid Cystic Carcinoma of nasal cavity (A) CT scan showing soft tissue mass (B) Gross specimen (C) Section shows the cribriform pattern gland like spaces filled with eosinophilic material (hematoxylin and eosin staining, x100, H&E) (D) IHC staining for CK 7 showing positivity (x400)
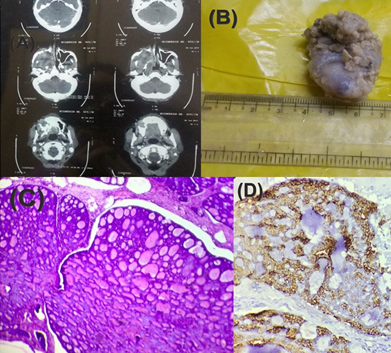
The overall histological features were in keeping with adenoid cystic carcinoma. Myoepithelial cells stained positive for p63, vimentin and ductal cells stained positive for CK 7 (Figure 6D). The diagnosis of adenoid cystic carcinoma of nasal cavity (Grade 2) was confirmed.
Case 7
(Pleomorphic adenoma of the parapharyngeal space)
A 55-year female presented to emergency with sudden unconsciousness which was followed by CT scan. Scans revealed ischemic CVA. On further physical examination, right oropharyngeal swelling was seen. History showed that the swelling lasted for 1 year. On digital palpation, swelling was firm, non-tender and bimanually palpable. She underwent CT scan again which revealed growth in the right parotid gland extending into the pre styloid compartment (Figure 7 A). A trans oral approach was done to remove the tumor mass. Grossly, mass measured 4 × 4 × 3 cm (Figure 7 B). Histologically, the section showed composed of chondromyxoid stroma with scattered glandular epithelial structures (Figure 7C) and was diagnosed as pleomorphic adenoma. On IHC, EMA (Epithelial membrane antigen) and CK 7 stained positive for the ductal. S-100 (Figure 7D) and p63 stained positive for the myoepithelial cells, thus, confirming the diagnosis.
Figure 7
Pleomorphic Adenoma of the Parapharyngeal space (A) CT scan showing growth in the right parotid gland (B) Gross specimen (C) Section shows chondromyxoid stroma with scattered glandular epithelial structures (hematoxylin and eosin staining, x100, H&E) (D) IHC staining for S100 showing positivity (x400)

Discussion
Tumors originating in the minor salivary glands are infrequent, and represent less than 20% of all salivary neoplasms.6,7,8 Pleomorphic adenoma (PA) is the most common benign MSGT, followed by myoepithelioma whereas mucoepidermoid carcinoma and adenoid cystic carcinoma (ACC) are the most common malignant tumors.9 MSGTs can be located anywhere in the upper aerodigestive tract, though the most frequently affected location is the oral cavity, and particularly the palate – where the concentration of minor salivary glands is greater. Such lesions also can be found in the cheek mucosa, in the region of the retromolar trigone, the lips, the oropharynx, or in the nasal cavities and paranasal sinuses, parapharyngeal space, trachea.10,11,12 Mucoepidermoid carcinoma occurred most often in the oral cavity whereas adenoid cystic carcinoma had a propensity for the sinonasal tract.5,13
In our case series, we have discussed pleomorphic adenoma, one in nasal cavity and two in parapharyngeal space and adenoid cystic carcinomas, three in nasal cavity and one in bronchus. MSGTs are common in fourth to sixth decade of life, more commonly affecting female patients, nearly 62%.14,15 In this study, four cases corresponded to female and three cases to male and belonged mostly from fourth to sixth decade of life. But we also have a younger age group of 10 years. Signs and symptoms can be related to tumor size and may vary according to the tumor site. Clinically, it is not possible to differentiate malignant and benign tumors of minor salivary glands. CT or MRI should be considered when assessing for presence of bony erosion or soft tissue and nerve involvement.16
Parapharyngeal space (PPS) resembles an inverted triangular pyramid with concave faces. The base of the pyramid is situated on the skull base and the apex is found where the posterior digastric muscles and the greater cornu of hyoid bone meet. Tumors in the parapharyngeal space are rare and constitute less than 0.5% of head and neck neoplasm.17 Approximately 70%–80% of these tumors are benign.18 Pleomorphic adenomas (PA) in the parapharyngeal space (PPS) can arise either de novo or may arise in the deep lobe of the parotid gland and extend through the stylomandibular tunnel into the PPS.19
World Health Organization (1972) defined PA as a well-defined tumor characterized by its pleomorphic or mixed appearance. There is intermixing of the clearly recognizable epithelial component with mucoid, myxoid and chondroid component.20 Pleomorphic adenomas of minor salivary glands exhibit increased myoepithelial cellularity with little or no stromal component which differs from those seen in major salivary glands. Immunohistochemical expression of cytokeratin and S-100 are used to confirm the diagnosis. The risk of developing carcinoma ex-Pleomorphic Adenoma from this benign tumor is 5% – 6% over twenty years.17,21 Bone is the predominant site for metastasis but spread to lungs, regional lymph nodes and liver has been documented.22
Adenoid cystic carcinoma (ACC) is a slowly growing, locally invasive tumor of salivary glands, with high tendency for perineural spread and bony invasion. A distinctive chromosomal translocationt (6;9) (q22-23; p23-24), which results in MYB-NFIB gene fusion, is found in adenoid cystic carcinomas.23 Here, the patient could not afford for the molecular study. The carcinoma cells originating from duct-type epithelial and myoepithelial cells of salivary glands have a dark blue nucleus, arranged in solid nests, tubular, cribriform patterns with pseudo-cystic spaces.24,25 The tumor is graded by the pattern of neoplastic cells arrangement. Cribriform is the most common histologic subtype. Currently, two different histopathological systems are used for grading of ACC. In the Perzin/Szanto system, ACC is classified into 3 grades- grade 1: predominantly tubular, no solid component; grade 2: predominantly cribriform, <30% solid; grade 3: Solid component>30%. In the Spiro system, the presence of more than 50% of solid components is considered high grade.26 In this study, the four adenoid cystic carcinomas belonged to grade 2 (predominantly cribriform).
In ACC, pulmonary metastases are the most common; however, metastases to the brain, bone, liver, kidneys, skin, abdomen and heart have also been reported.27,28,29,30 Immunohistochemical staining shows that tumor cells with myoepithelial differentiation express S100 and p63.31 The interspersed ductal epithelial cells express CK7, CD117.31 Among various treatment modalities, combined primary excision with postoperative radiotherapy appears to achieve more satisfactory local control when compared to either surgery or radiotherapy alone.32,33 Chemotherapy appears to be ineffective in the treatment of adenoid cystic carcinoma. Long-term follow-up is necessary because of the high incidence of local recurrence and distal metastasis.
In our study, the patients are still under follow up.
Conclusion
Minor salivary gland malignancies are rare tumours with varied histology and can occur in any age group including younger patients. Outcome is variable and is influenced by tumour type and anatomical location of the tumour. Preoperative radiological assessment is very essential for the head and neck surgeon to know the pattern of spread and to remove the tumour completely so that there is least chance of recurrence. Long term survival outcome is determined mainly by systemic spread of disease.
