- Visibility 81 Views
- Downloads 6 Downloads
- DOI 10.18231/j.jdpo.2019.051
-
CrossMark
- Citation
An audit of Preanalytical errors as a quality measure in central clinical laboratory of Rural Tertiary Care Hospital in Eastern Uttar Pradesh
- Author Details:
-
Vaishali Dhananjay Kotasthane
-
Jaya Singh
-
Dhananjay Shrikant Kotasthane *
Introduction
Laboratory medicine is the important stake holder in the quality management services provided to the patient for better clinical outcome and no one can deny that generating Quality lab report is the soul of Laboratory medicine. Quality lab reports not only act as a facilitator to treating physicians in their process of decision making but also prove to be cost effective in long range for the lab investigator. The responsibility of generating quality reports involves varied stakeholders ranging from lab technicians, nursing staff, consultants, lab managers or the respective institute itself to work upon providing error free reports to their patients. Although in India, we have standardized ISO15189 accreditation for clinical laboratories certified by National accreditation agency i.e NABL [National Board for Laboratory Accreditation] that has prescribed all relevant guidelines for quality control and quality assurance in a clinical laboratory, but since it is not a mandatory requirement for every clinical Lab to be NABL accredited/ISO certified, this weakens the backbone for quality in lab medicine.
Every laboratory sample undergoes processing through three phases[1] Pre-analytical phase[2] Analytical phase[3] Post-analytical phase. Analytical phase lies in the least vulnerable phase if viewed in perspective of generating errors due to increased automation in technology and least human action involved. It is now difficult to find errors in this phase until any breach in the machinery has occurred, this way burden shifts towards the pre-analytical phase related to proper sample collection, handling of sample, spillage or leakage etc. This phase depends on less mechanized activity and more human activity dependent involving laboratory technicians and nursing staff resulting in human errors. As nearly 60% of the testing process is centered on pre-analytical phase, pre-analytical errors account for two thirds of all laboratory errors.[1] Most studies demonstrated that a large percentage of laboratory errors occur in the pre and post analytical phases, with fewer mistakes occurring in analytical phase.[2] In one study, pre-analytical error was mentioned as 46-68%.[3] In another study, errors occurred during pre-analytical phase were as high as 61.9%.[4] The common types of pre -analytical errors reported in different studies were: ordering tests on the wrong patient, misidentifying the patient, missing sample and/or test request, contamination from infusion route, hemolyzed, clotted, and insufficient samples, inappropriate containers, improper labeling of containers, inappropriate blood toanticoagulant ratio, and inappropriate transport and storage conditions.[4]
Quality Indicators are useful performance monitoring tools for the pre analytical phase of the testing process.[5] The magnitude of the effect of these errors on patient care is not negligible since information provided by clinical laboratories affects up to 60-70% of clinical decisions.[6] Error in this phase may also lead to improper diagnosis which will further affect the cost of treatment for the patient. About 60%–70% of clinical decisions regarding admission, prescription, and discharge are based on laboratory results.[7]
The criteria for the choice of quality indicators have been widely accepted by health organizations, and can be grouped into three conceptual areas: 1) significance, 2) scientific base, and 3) the possibility of measurement, which are elaborated in detail depending on where they are applied.[8]
For a good laboratory practice, Quality assurance provides management and attention at every step beginning from aseptic precautions, followed by sample collection, order form match, transportation to the central clinical laboratory until the analytical phase begins. The use of nationally standardized testing algorithms, as well as standard operating procedures and QC procedures, is vital to assuring quality of testing.[9] Guidelines for quality assurance system should be maintained in the procedure manuals used by all the technicians working in the laboratory. Better compliance and coordination is necessary in order to increase the effectiveness and accuracy of laboratory result.
There are many fundamental justifications for improving the laboratory reports, Quality assurance falls first in the category which pays attention to every step of processing, second is the quality control which maintains the laboratory guidelines for any testing. In order to follow the above steps, not only laboratory technicians should be trained but also nursing staff in the wards should also be competent enough, and above all every ward or sample collection area should have the standardized operative procedures (SOPs) by Central Clinical Laboratory that mentions the do’s and don’ts of sample collection effectively monitored by lab managers. Thus, it is need of hour that each lab should have their own quality checks to monitor all possible errors to produce error free lab reports. With this background in mind, we aim to address this issue through our study of audit of pre-analytical errors in Central Clinical Laboratories.
Aims and Objectives
To identify type of Pre-analytical errors occurring in OPD and IPD blood samples received in Central Clinical Laboratory.
To recommend solutions to overcome the errors to improve quality of Central Clinical Laboratory reports.
Material and Methods
Type of study
This study was a non-participatory observational study for a period of two months i.e 6/7/2019 to 6/9/2019. After obtaining clearance from Institutional Human Ethical committee, this cross sectional study was undertaken in Central Clinical Laboratory (CCL) in the Hospital premises of Heritage Institute of Medical Sciences.
Study design
The blood samples collected in OPD and IPD for CCL were included in the study.
After sample collection, needle and syringe were destroyed according to Biomedical waste management guidelines followed by our hospital. Needle stick injuries were observed and taken care of, if any according to Guidelines laid down by Hospital infection control committee of Institute.
Study population
Patient who gave blood samples in OPD and IPD for lab tests were included in the study.
Sample size
Blood samples collected during study period of two months i.e.From 6/7/19 to 6/9/19 were included in study.
Selection criteria
The blood samples were received in anticoagulated (for whole blood and Plasma) and non- anticoagulated (Serum) vaccutainer from OPD and IPD after proper aseptic precautions. Vaccutainer used in institute were Novacpolymed company for both serum and anticoagulated blood sample.
Inclusion criteria
All Blood samples collected from OPD and IPD patients for CCL investigations
Exclusion Criteria
Clinical Pathology samples i.e. urine, body fluids (pleural, peritoneal, pericardial, synovial, CSF), semen and stool samples were excluded from our study.
Confidentiality was maintained throughout the study
Statistical tests
Percentage was used for descriptive statistics to explain the distribution. Pie diagram and bar diagrams were used for simple data interpretation by excel software. For continuous variables which follow normal distribution curve, parametric test like Chi-square was applied to look was any significant difference in variables. P value calculated with one degree freedom and p value <0.05 was considered to be significant.
Results
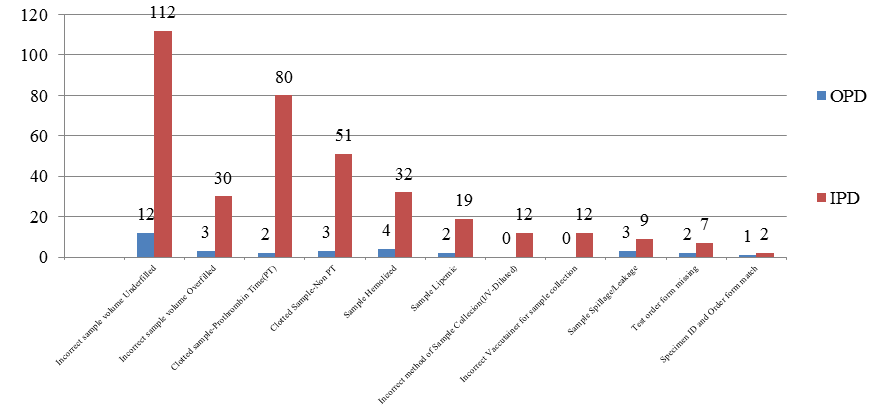
Total number of blood samples received for OPD(n=3658) and IPD (n=9946) patients together in the study period of two months were 13604. Out of 13604 samples, overall pre-analytical errors accounted for 2.93%(398 samples).[[Table 1][Figure 1]]
| S.No. | Pre-analytical Errors | Errors in OPD samples (n=3658) | Errors in IPD samples (n=9946) | % of individual error in Total error (n=398) | % of error in total samples (n=13604) | |||
| 1 | Incorrect sample volume | Underfilled | 12 | 112 | 124(31.16% | 157 (39.44%) | 0.91% | 1.15% |
| Overfilled | 03 | 30 | 33(8.28%) | 0.24% | ||||
| 2 | Clotted sample | Prothrombin Time(Citrate) | 02 | 80 | 82(20.60%) | 136(34.17%) | 0.60% | 0.99% |
| Non PT (EDTA) | 03 | 51 | 54(13.57%) | 0.39% | ||||
| 1. 3 | Hemolyzed Sample | 04 | 32 | 36(9.05%) | 0.26% | |||
| 2. 4 | Lipemic Sample | 02 | 19 | 21(5.28%) | 0.15% | |||
| 3. 5 | Incorrect method of Sample Collection(I/V-Diluted) | 00 | 12 | 12(3.02%) | 0.08% | |||
| 4. 6 | Incorrect Vaccutainer for sample collection | 00 | 12 | 12(3.02%) | 0.08% | |||
| 5. 7 | Sample Spillage/Leakage | 03 | 09 | 12(3.02%) | 0.08% | |||
| 6. 8 | Test order form missing | 02 | 07 | 09(2.26%) | 0.06% | |||
| 7. 9 | Specimen ID and Order form match | 01 | 02 | 03(0.74%) | 0.02% | |||
| Total errors | 32 | 366 | 398 (100%) | 2.93% | ||||
| Percentage of OP/IP errors in total error of 398 samples | 8.05% | 91.95% | 398(100%) | |||||
| Total blood samples received (n) | 3658 | 9946 | 13604 | |||||
| Overall percentage of pre-analytical errors in total samples(n=13604) | 0.87% | 3.68% | 2.93% |

Table 1 illustrates that all Pre-analytical errors were more common in IPD blood samples contributing for 3.68% out of 9946 IPD samples as compared to OPD samples which accounted for 0.87% errors out of 3658 OPD blood samples. Thus, in total 398 pre-analytical errors identified, IPD errors contributed for 91.95% as compared to 8.05%. This difference in error was statistically significant (Chi sq. value =74.09 and value of p<0.05). The comparative errors between OPD and IPD samples are illustrated in [[Figure 2]]
| S. No | Type of Errors | EDTA+CITRATED (Haematology) | Plain+Fluoride (Biochemistry) | Plain (Serology) | Total |
| 1. | Incorrect Vaccutainer for sample collection | 4( 33.3%) | 8(66.7% ) | - | 12 |
| 2. | Incorrect Sample Volume-Underfilled | 34(27.4%) | 70(56.5%) | 20(16.1%) | 124 |
| 3. | Incorrect Sample Volume-Overfilled | 33(100%) | - | - | 33 |
| 4. | Lipemic Sample | - | 13(61.9%) | 8(38.1%) | 21 |
| 5. | Hemolysed Sample | 11(30.6%) | 20(55.6%) | 5(13.9%) | 36 |
| 6. | Incorrect method of Sample Collection (I.V /diluted samples) | 10(83.3%) | 02(16.7%) | - | 12 |
| 7. | Clotted Sample | 129(94.9%) | 7(5.1%) | - | 136 |
| Total | 221(59.09%) | 120(32.08%) | 33(8.82%) | 374 |
Error 1: Incorrect sample volume collected in vaccutainers
From [Table 1], the highest frequency of error was found to be incorrect sample volume 1.15% (157/13604) collected in vaccutainers in total samples and contributed for 39.44% of total errors(157/398). It consists of both underfilled and overfilled sample collection. Out of total 398 sample errors, errors of underfilled vaccutainers were more common (31.16%) than the overfilled (8.28%).[Figure 1] [Table 2] showed distribution of underfilled error was predominant with Plain vaccutainer for biochemistry and serology (72.6%)whereas hematology accounted for (27.4%) out of total 124 underfilled samples and overall accounted for 0.91% in total samples. In contrast, overfilled vaccutainers accounted for 0.24% of total samples. Among vaccutainers, this error was seen exclusively with citrated vaccutainer (Prothrombin time) and EDTA especially in IPD samples for hematology. ([Table 2])
Error 2: Clotted sample
Clotted sample was the second highest pre-analytical error 0.99% (157/13604) in total samples received in study period and accounted for 34.17%(136/398) of all the errors in total error samples. In this, clotted sample for Prothrombin time (citrated) accounted for 20.60% and remaining (EDTA+Fluoride) accounted for 13.57% in total error samples ([Table 1]). On a daily basis, Central Clinical Laboratory at HIMS receives 6 to 8 samples for Prothrombin time (PT)for analysis; out of which 0-3 samples per day were clotted due to overfilled samples. As PT sample requires exact proportion of anti-coagulant to blood (1:9), any excess of this proportion (eg: 1.8 ml blood required to be poured in 2ml blue coloured PT vaccutainer) results in clotting. This error was again more common with IPD samples ([Figure 1]). As mentioned previously, this error was predominant in Hematology blood samples collected in EDTA and PT samples(94.9%) and remaining (5.1%) were seen in biochemistry for fluoride vaccutainer out of total clotted samples (n=136)([Table 2]).
Error 3: Hemolyzed Sample
The error of hemolyzed samples accounted for 0.26% (36/13604) of total samples and 9.05% (36/398) of total error in our study. It could be observed from [Table 2] that hemolyzed samples were commonly seen with plain vaccutainer collected for Biochemistry and Serology together accounting for 69.5%(25/36) and also predominant in IPD samples as compared to OPD samples.([Figure 2])
Error 4: Lipemic samples
The lipemic samples accounted for 0.15% (21/13604) of all the samples and 5.28% (21/398) of error in total error in our study, also more common in IPD samples ([Figure 2]). This error was common for samples collected in plain vaccutainer for biochemistry 61.9%(13/21) and serology 38.1%(8/21). [[Table 2]]
Error 5: Incorrect method of sample collection
Improper method of sample collection eg-sample collected improperly from I.V cannula in IPD patients resulting in diluted sample accounted for 0.08%(12/13604) of total samples and 3.02% (12/398) of error out of total errors and was seen predominantly with Hematology samples(83.3%). [[Table 2]]
Thus, from [Table 2] it was evident that overall redistribution of common pre-analytical errors showed highest errors in hematology samples and accounted for 59.09%, followed by biochemistry (32.08%) and serology (8.82%) in 374 sampling errors.
Error 6 & 7 : Incorrect vaccutainer for sample collection & sample spillage/leakage
These pre -analytical errors contributed for 0.08% of total samples and 3.02%(12/398) each in total errors. The error of incorrect vaccutainer for sample collection was seen exclusively in IPD samples([Table 1]). The sample spillage/leakage was seen predominantly in IPD samples during transportation transit from ward to central laboratory.
Miscellaneous errors
The errors of test order form missing with sample and specimen ID and order form mismatch was found to be very less and accounted for 0.06% and 0.02% of total samples and accounted for 2.26%(9/398) and 0.74%(3/398) of total errors respectively.
Discussion
Quality lab reports are integral part of quality patient care. In this technology driven era, modern automated analyzers have reduced analytical errors and burden of error got redistributed to human driven pre-analytical and post -analytical phases. As nearly 60% of the testing process in clinical labs is centered on pre-analytical phase, pre-analytical errors account for two thirds of all laboratory errors[1]. In literature, pre-analytical error ranged from 46-68% of total lab errors of all phases[3]. In another study, errors occurred during pre-analytical phase was noted to be 61.9%.[4]
In our study, frequency of Pre-analytical errors accounted for 2.93%.in total samples received in the two months duration of study period. The frequency of pre-analytical error in other studies ranged from 0.38% to 5.20% ([Table 3] ) and difference in our study with these studies was statistically significant (X2 values for following respective studies when compared with our study w ere 1892,1110,404,230 with one degree of freedom respectively with (p<0.05).[3],[4],[10],[11]
| S.No | Studies | Frequency of Pre-analytical errors |
| 1 | Present study | 2.93% |
| 2 | Narang 3 | 0.38% |
| 3 | Arul4 | 0.43% |
| 4 | Upreti 11 | 1.34% |
| 5 | Bhuyar12 | 5.20% |
The common variables of pre-analytical errors studied in different studies were inadequate samples, misidentification, clotted samples, and hemolyzed samples. According to the various studies mentioned in literature, frequency of pre-analytical errors for different variables noted were - incorrect number of samples 2.3%, sample not received 2.9%, hemolyzed sample 0.8%, clotted sample 0.55%.[11],[13],[14],[15] [Table 4] shows frequency of common pre-analytical errors noted in various studies.
| Pre-analytical errors | Present study | Upreti 10 | Bhuyar 12 | Gupta M.1 | Arul4 | Englezopoulon 16 |
| Incorrect sample volume | 1.15% | 0.19% | 1.8% | 3.03% | 0.2% | 0.09% |
| Clotted sample | 0.99% | 0.13% | - | 1.03% | 0.12% | 0.16% |
| Hemolysed sample | 0.26% | 0.09% | 7.8% | 3.33% | 0.03% | 0.15% |
| Incorrect method(diluted sample) | 0.08% | 0.04% | 0.04% | 2.1% | 0.02% | 0.07% |
| Specimen ID & order form match | 0.02% | 0.35% | - | 1.23% | 0.02% | 0.24% |
In our study, frequency of pre-analytical errors in IPD samples received were (3.68%) and found to be more than OPD (0.87%) in respective of total IPD and OPD samples. Thus, the contribution of pre-analytical error in IPD sample was 91.95% as against OPD (8.05%) in total error encountered. This difference in error was statistically significant (p<0.05). Similar findings were noted by Arul et al.[12] The reason behind this variation might be variable paramedical staff involved in sample collection, increase transport transit time, lack of motivation, trainee staff involved in sample collection in ward during peak hours instead of skilled staff, inadequate staff against excess work load, variable skill of staff, poor clinical condition of IP patients. In our study, blood sample collected for hematology contributed for 4.90% error in total hematology sample received biochemistry 1.57% and serology (microbiology) 2.25% in total samples received in respective sections. Another foreign study from Greece showed Hematology error to be 0.065% and serology to be 0.139%, the difference in percentage of error might be due to more modernization technique and trained and adequate staff in western and European countries[16].
Incorrect Sample Volume
In the present study, inadequate sample volume was the highest frequency of error noted and accounted for 1.15% of total samples received during study period. From Table 4, it could be noted that this error showed wide range from 0.19 to 3.03% in Indian studies [1],[4],[12],[10] where as foreign study from Greece noted this error to be only 0.09%.[16] This difference in Indian and foreign study may be due to availability of trained staff to minimize human error in sample collection. Under inadequate samples, error of underfilled vaccutainers accounted for 0.91% in total samples and contributed for frequency of 31.16% in total errors identified and also more common in IPD samples. The error of underfilled vaccutainers was noted predominantly with paediatric samples. This could be due to difficulty to collect samples in pediatric sick patients requiring trained paramedical staff or when number of tests advised per patient were more requiring sample to be collected in every vaccutainer (i.e. EDTA/Plain/fluoride) but the blood sample collected was not proportionally adequate. The error of over filled samples was reported to be 0.24% of total samples in the present study. This error of overfilled vaccutainers could occur when single test was advised and blood sample was overtly collected and inadequate knowledge of paramedical staff regarding quantity of sample collection.
Clotted samples
The second common pre-analytical error in our study was clotted sample accounting for 0.99% of total samples. The error of clotted sample ranged from 0.12% to 1.03% as documented in different Indian studies and one foreign study[[Table 4]].The reason for this could be improper mixing of blood with anticoagulant like EDTA and citrate for PT) in the vaccutainer after blood collection. In hematology, this was the commonest error in our study contributing for 94.9% in all clotted sample. This error again was noted more commonly in IPD samples. This was the most frequent reason for rejection with the highest frequency of rejection in pediatric tube in one study.[17] Other causes could be leaving blood in syringe for long hours before placing in tubes as due to absence of anticoagulant blood from a vein may coagulate easily.
Hemolyzed and Lipemic sample
Hemolyzed samples accounted for 0.26% in total samples received in our study. Frequency of error due to hemolyzed samples showed a varied range from 0.03 to 7.8% in different Indian studies as mentioned above in [Table 4]. Haemolysis may interfere with the test method. The amount of interference will depend on the degree of haemolysis and particular test method applied. This error was common in biochemistry (55.6%) and serology(13.9%) of all the hemolyzed samples received. As noted in literature, haemolysis was the common cause of specimen rejection in laboratories, hence leads specimen to be redrawn[18].
Lipemic samples accounted for 0.15% of error in total samples in our study whereas Chawla et al and Englezopoulou et al noted lower frequency of lipemic sample at 0.07% and 0.09% respectively.[19],[16] This might be due to non-communication to patient to remain fasting before sample collection or due to increased incidence of obesity due to dietary and lifestyle changes in this diabetic and pre -diabetic capital of world.
Other errors noted in our study were - Incorrect method of sample collection especially in IPD patients resulting in diluted sample (I/V-Diluted) and accounted for 0.08% of total samples in our study. Other studies noted this error from 0.02% to 2.1%[[Table 4]]. This error can be corrected by drawing blood from opposite arm[20]. Other studies have reported diluted samples as a cause of rejection in paediatric age group. [10],[21],[22]
Other miscellaneous errors encountered in our study were- Incorrect vaccutainer for sample collection (0.08%), Sample Spillage/Leakage (0.08%), Test order form missing (0.06%), Specimen ID and Order form mismatch (0.02%). Although error due to Sample Spillage/Leakage(0.08%) and misidentification (0.02%) were less in our study, another Indian study noted the frequency at 0.33% and 1.23% respectively.[1] Also, Englezopoulou et al noted misidentification to be 0.24%.[16]
Thus, variables of pre-analytical errors showed variation in different Indian studies.
As this study is audit of pre-analytical errors, to overcome these errors following suggestions are suggested for improvement in the quality of lab reports:
As pre-analytical phase is human driven, chances of reducing error in this phase lies in good laboratory practices which includes frequent training of paramedical staff involved in sample collection, transportation, correct data entry and verification, increased use of automation technology, technological support in the form of lab management system, barcodes for simplification and proper tracking of specimen, proper interpersonal communication, Standard operative procedures(SOPs) written in different collection areas and last but not the least is Lab accreditations.[3],[13],[19]
Thus, greatest impact on overall improvement could be achieved by focusing on the pre-analytical processes in which most “gross” errors occur due to human factor, the errors that can lead into adverse events or the risk of adverse events for patients.[23]
Sugesstions
The present study being audit of pre-analytical errors, suggestion to rectify avoidable errors becomes mandatory.
Quality assurance in a laboratory brings about accuracy and precision in laboratory. Erroneous report not only halter the diagnosis of physicians but also decreases interest of patient towards the hospital working, as it becomes very cost effective for them to undergo a test several times which further leads to loss of Patient interest. In spite of well- equipped laboratory set-up, pre-analytical phase still falls under the category where Quality Control depends upon the skills and sincerity of the Lab technician and Nursing staff. There are many factors that lead to generating pre-analytical errors, common being error occurring during specimen collection, handling, transportation especially in Inpatients and physical condition of the patient. In literature, it has been documented that minimizing the errors at any step in the pre-analytical phase, a laboratory can improve quality of analytical result, which further reduces re-collected specimen, and brings an improved patient management.[21]
In order to produce accurate test results there are many steps where quality assurance plays a major role and guarantees error free set-up mentioned in literature [24]. They are as follows:
1. Proper Patient Identification is critical for:
Test order form must be present
Specimen ID and order form match must be done
Before performing any venipuncture patients physical condition and amount of blood required for analysis needs to be kept in mind
2. Every Hospital Management system should pay great attention towards proper training and education of Skilled Laboratory Technicians and especially Paramedical staff in wards for accuracy and precision of Laboratory reports.
3. Responsibility not only lies over the Hospital Management system, but also over the Laboratory. This could be followed by making specific guidelines those are readable to everyone in their local language, in the form of pamphlets or Notice board at the area of sample collection especially in wards as our study has shown that errors are occurring more in IPD samples with statistically significant from OP errors.These precautions will further contribute to reduce the number of error and provide quality test reports to the patient.
4. Identification of venipuncture site, correct positioning of Patient and its preparation should be strictly dependent upon Physical condition of the Patient.
5. For any Hematological sample collection, EDTA should be used as a standard anticoagulant; and blood filled tubes should be immediately mixed by complete inversion this way Anticoagulant is distributed homogenously.
6. Mixing of tube: Clotting could be prevented by proper mixing of specimen collected with EDTA.
7. Specimen storage and Transportation: Any specimen collected either from OPD or IPD should be sent to Laboratory without delay so as to avoid errors like Clotted, Hemolysed and Lipemic sample.
8. Specimen integrity could be maintained by looking over the following parameters:
Sample labeling
Correct sample volume
End to end mixture of sample within the tube before analysis
Should always keep the specimen as well as tubes at room temperature, not at higher temperature to avoid errors.
Conclusion
The present study concluded that pre-analytical errors are avoidable by proper training and education to the Laboratory technicians and Nursing staff which are the main stakeholders in good laboratory practices in generating quality lab reports. In order to maintain efficacy of the lab results, it is important to follow guidelines which should be formulated by each lab managers based on SOPs prescribed by accreditation agencies from time to time. This study can act as a pilot study and scaffold for future prospective study to formulate policies to monitor preanalytical errors in Central Lab. The lesser the number of pre-analytical errors, the more accurate and précised will be the laboratory results.
Acknowledgements
We acknowledge ICMR for approving this study proposal under short term studentship programme. We also acknowledge cooperation of technical staff, CCL for helping in collecting data for this study.
Source of Funding
None.
Conflict of Interest
None.
References
- M Gupta, D Yadav, P Sharma. Identification of Pre-analytical Errors in the Clinical Laboratory of North Indian Tertiary Care Hospital. Biochem Physiol 2015. [Google Scholar]
- S Naz, Arshadm Sadaruddin, A .. Pre-analytical Errors and their Impact on Tests in Clinical Laboratory Practice. Pak J Med Res 2012. [Google Scholar]
- V Narang, H Kaur, P Selhi, N Sood, A Singh. Preanalytical Errors in Hematology Laboratory-An avoidable Incompetence. Iran J Pathol 2016. [Google Scholar]
- P Arul, M Pushparaj, K Pandian, L Chennimalai, K Rajendran, E Selvaraj. Prevalence and types of pre-analytical error in hematology laboratory of a tertiary care hospital in South India. J Lab Physicians 2018. [Google Scholar]
- D Grecu, D Vlad, D Victor. Quality indicators in the Preanalytical phase of testing in a Stat Laboratory. Lab Med Feb 2014. [Google Scholar]
- H Tadesse, K Desta, S Kinde, F Hassen, A Gize. Errors in the Hematology Laboratory at St. Pauls Hospital Millenium Medical College. BMC Res Notes 2018. [Google Scholar]
- M Plebani. Errors in Clinical Laboratories or errors in Laboratory medicine. Clin Chem Lab Med 2006. [Google Scholar]
- E Kelley, J Hurst. Health care quality indicators project conceptual framework paper. OCED. Int J Qual Health Care 2006. [Google Scholar]
- . UNAIDS/WHO. Guidelinesfor organizing national external quality assurance schemes for HIV serological testing. Geneva: UNAIDS/WHO; 1996.. . [Google Scholar]
- S Upreti, R Bansal, N Jeelani, V Bharat. Types and frequency of pre-analytical error in hematology lab. J ClinDiag Res 2013. [Google Scholar]
- S Shahangian, R S Synder. Laboratory medicine quality indicators. Am J Clin Pathol 2009. [Google Scholar]
- K B Bhuyar. A study of Pre-analytical errors in a hospital based clinical biochemistry laboratory. Internl J Biotech Biochem 2017. [Google Scholar]
- R Hawkings. Managing the pre and post analytical phases of the total testing process. Ann Lab med 2012. [Google Scholar]
- G Lippi, J J Chance, S Church, P Dazzi, R Fontana. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med 2011. [Google Scholar]
- G Lipi, G Banfi, M Buttarello, F Ceriotti, M Davis, A Dolci. Recommendations for detection and management of unsuitable samples in clinical laboratories. Clin Chem Lab Med 2007. [Google Scholar]
- A Englezopoulou, M Kechagia, R Chatzikiriakou, M Kanellopoulou, M Valenti, F Masedu. Pre Analytical errors as quality indicators in clinical laboratory. Austin J Public Health Epideml 2016. [Google Scholar]
- P J Howanitz. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med 2005. [Google Scholar]
- A Pfutzner, C Schipper, S Ramljak, F Flacke, J Sieber, T Forst. Evaluation of the Effects of Insufficient Blood Volume Samples on the Performance of Blood Glucose Self-Test Meters. J Diabetes Sci Technol 2013. [Google Scholar]
- R Chawla, V Goswami, D Tayal, V Mallika. Identification of the types of preanalytical errors in the clinical chemistry laboratory: 1 year study of GB Pant Hospital. Lab Med 2010. [Google Scholar]
- A K Stankovic, E Delauri. Quality improvements in the Preanalytical Phase: Focus on Urine Specimen Workflow. MLO Med Lab Obs 2008. [Google Scholar]
- T F Ashavaid, S V Dandekar, S Khodaji, M H Ansari, A P Singh. Influence of method of specimen collection on various preanalytical sample quality indicators in EDTA blood collected for cell counting. Ind J Clin Biochem 2009. [Google Scholar]
- J E Favaloro, D M Funk, G Lippi. Pre-analytical variables in coagulation testing associated with diagnostic errors in hemostasis. Lab Med 2012. [Google Scholar]
- G Lippi, G C Guidi. Readers Response and Authors Reply to Laboratory Results That Should Be Ignored. Med Gen Med 2006. [Google Scholar]
- . Wayne PA. In: Procedures for the collection of diagnostic blood specimens by venipuncture;approved standard : National Committee for clinical Laboratory standards Document, 5thed ,2003 ,Vol 23; No 32;H3-A5.. . [Google Scholar]
- Introduction
- Aims and Objectives
- Material and Methods
- Type of study
- Study design
- Study population
- Sample size
- Selection criteria
- Inclusion criteria
- Exclusion Criteria
- Statistical tests
- Results
- Error 1: Incorrect sample volume collected in vaccutainers
- Error 2: Clotted sample
- Error 3: Hemolyzed Sample
- Error 4: Lipemic samples
- Error 5: Incorrect method of sample collection
- Error 6 & 7 : Incorrect vaccutainer for sample collection & sample spillage/leakage
- Miscellaneous errors
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
