Author Details :
Volume : 4, Issue : 1, Year : 2019
Article Page : 27-31
https://doi.org/10.18231/2581-3706.2019.0005
Abstract
Introduction: The aim was to study the expression of p57(KIP2) immunohistochemical marker in first trimester abortion specimens of molar and non-molar pregnancies.
Materials and Methods: The study design was a cross-sectional study conducted in the Department of Pathology of a teritiary health care centre in South India. 100 cases of first trimester abortion specimens of molar and non-molar pregnancies were studied.The study duration was two years. The IHC used is p57(KIP2) which is a mouse derived monoclonal antibody and its expression in decidual and villous tissue of abortion specimens were observed.
Result: Of 100 cases of first trimester abortion specimens 25 cases were complete mole, 17 were partial mole and rest 58 cases were normal abortions. p57 gene is paternally imprinted and maternally expressed, and the presence of its protein product serves as an useful adjuvant marker for the nuclear maternal genome. Regarding the expression of p57(KIP2) immunomarker in partial mole(PM) diagnosed previously by H&E 88% expressed positive results. These findings were highly statistically significant. The results of p57 IHC marker expression in cases of complete mole diagnosed previously by H&E, 96% expressed negative results which confirmed the complete mole diagnosis.
Conclusion: The study of p57 immunohistochemistry in abortion specimens has proved to be an ancillary diagnostic tool in the diagnosis of complete mole. The sensitivity and specificity of p57 were very high in diagnosing complete mole from its close mimic partial mole in early first trimester abortions. The sensitivity and specificity were 96% and 88% respectively.
Keywords: Hydatidiform mole (HM), Immunohistochemistry (IHC), Complete mole (CM), Partial mole (PM).
Gestational trophoblastic disease are a group of benign and malignant entities, where the hydatidiform mole is the most common, and can be divided into complete hydatidiform mole and partial hydatidiform mole.[1] Hydatidiform mole is an abnormal placenta with villous hydrops and trophoblastic proliferation[2] Complete mole involves all the chorionic villi and has a diploid karyotype.[2] Partial mole is an hydatidiform mole with both normal villi and hydropic villi and focal trophoblastic proliferation. These lesions have triploid karyotype.[2] The distinction is made between complete and partial mole based on clinical, morphologic and genetic difference.[3] A complete mole is diploid, generally has a 46XX or a 46XY karyotype and is entirely androgentically derived. A partial mole usually contains an extra haploid set of paternally derived DNA and has 69 chromosomes.[4]
However the histologic criteria are still questionable and histologic evaluation alone is prone to great interobserver variability.[5] The risk of persistent disease and choriocarcinoma is more with complete mole.[6] Persistent trophoblastic disease develop after a complete mole in 10% to 30% cases and after partial mole in 0.5% to 5% cases.[7][8]Choriocarcinoma arises in 3% of complete mole and is rare with partial mole.[9] Therefore distinguishing the molar pregnancy especially the more dangerous complete mole from its mimics during early gestation period is an important factor in management and prognosis. More challenges are experienced nowadays with advanced number of prenatal screening techniques that provide earlier clinical recognition and termination of abnormal pregnancies.[4] p57(KIP2) is the protein product of the paternally imprinted but maternally expressed gene, (CDKNIC) located on chromosome no 11p15.5.[10] Because complete mole lack a maternal genomic component they are not expected to express imprinted genes that are normally expressed by the maternal allele and immunohistochemical analysis for p57(KIP2) has been shown to be a valuable tool in the diagnosis of a complete mole.[8]
Low or absent p57(KIP2) expression likely plays an important role in the abnormal proliferation and differentiation of trophoblasts in tetraploid complete mole. In the villous tissue it is absent in synctiotrophoblast and strongly expressed in cytotrophoblast, villous mesenchyme and intervillous or intermediate trophoblast.[11] Since complete mole lacks a maternal genome this gene is completely absent or weakly expressed. Partial mole, hydropic abortus show positive expression with p57(KIP2) immunomarker. p57 IHC marker has an advantage of differentiating hydropic abortus from complete mole, a distinction not possible by ploidy analysis. Both complete mole and hydropic abortions on ploidy analysis show diploid karyotype which is well differentiated by p57immunohistochemistry.[5]
The study design was a cross-sectional study conducted in the Department of Pathology of a tertiary health care centre in South India from January 2015 to june 2016. First trimester abortion specimens received in the department of pathology during the study period along with blocks of retrospective cases from previous two years were included. Abortion specimens include complete mole, partial mole and non- molar products of gestation. Ectopic abortions, second trimester abortions and those abortions with villous tissue were excluded. Formalin fixed paraffin embedded tissue blocks of abortion specimens were stained by routine Haematoxylin and Eosin on sections. Immunohisto-chemical studies were performed using 4 micrometre formalin fixed, paraffin-embedded sections. The IHC used is p57(KIP2) which is a mouse derived monoclonal antibody (clone:25B2) and its expression in decidual and villous tissue of abortion specimens were observed. Maternal decidua and intermediate trophoblast served as internal positive control. Syncytiotrophoblast served as internal negative control. The H/E sections were observed for the histopathological changes in villous tissue. The nuclear p57(KIP2) staining were observed in decidual and villous tissue of abortion specimens. The staining was interpreted as a scoring pattern. IHC pattern was interpreted as done in study by Dr. Fukunaga.[4] Nuclear positivity was counted in cytotrophoblast and villous stromal cells.
|
No staining |
Score 0 |
|
1%-10% positive cells |
Score1+ |
|
10%-50%positive cells |
Score2+ |
|
>50% positive cells |
Score3+ |
0,1+ were condidered as negative expression and 2+, 3+ were considered as positive expression.
The calculated data were entered with excel sheets and analysed using Epinfo 7 and SPSS trial version
Total 100 cases of abortion specimens both molar and non-molar were observed. The age of the patients ranged from 18 to 42 yrs with frequent age group being 20 to 30 yrs. Among the 100 abortion specimens 25 were complete mole 17 were partial mole and rest were normal abortion specimens. Majority of study population were in the gestational age of 8-10 wks. Majority of patients with molar abortion were multiparous and multigravidae whereas majority of study population with normal abortion specimens were primigravida.
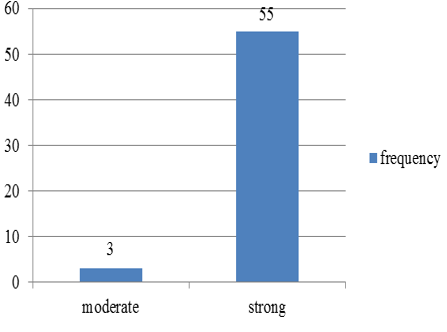
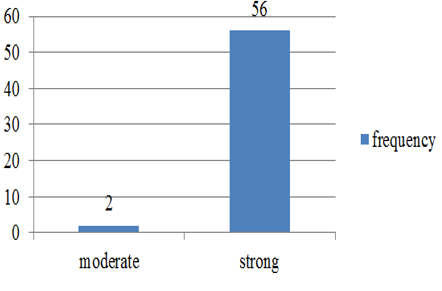
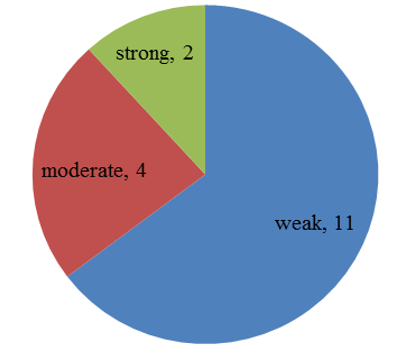
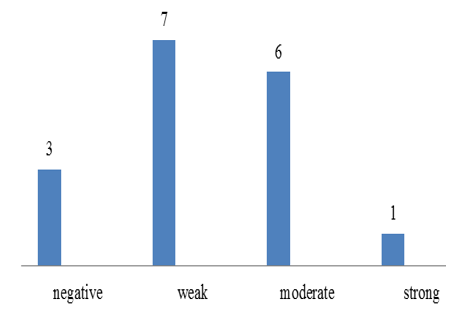
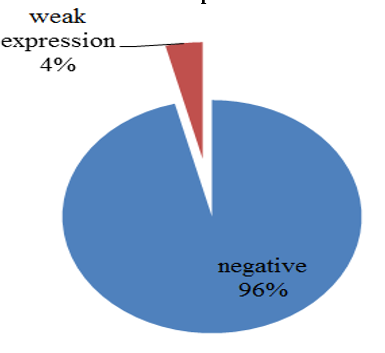
p57(kip2) expression in cytotrophoblast and villous stromal cells were studied in molar and non- molar abortions. 96?ses of complete molar abortions showed negative expression in cytotrophoblast and 82% cases of partial mole showed positive [removed]Table 1, Chart 4). All cases of partial mole showed positive expression in villous stromal cells with majority cases (64%) showed weak intensity of staining. (Chart 3) 96% cases of complete mole showed negative expression in villous stromal cells (Chart 5).Out of 25 cases of complete mole 24 cases showed negative expression and was statistically significant. Similarly out of 17 cases of partial mole 15 cases showed positive expression which was statistically significant and 2 cases showed negative [removed]Table 2). 58 cases of normal abortions cases were studied and majority of cases (96%) showed strong intensity staining with p57(KIP2) and did not show any proliferation of cytotrophoblasts (Chart 1,2).
The sensitivity and specificity was high for p57(KIP2) in diagnosing complete mole and partial mole which agreed with studies stating that it can be used as a ancillary diagnostic tool replacing ploidy analysis in differentiating complete mole and its mimics. The sensitivity and specificity of p57(KIP2) in distinguishing complete mole from its mimics were 96% and 88% respectively. The sensitivity and specificity of p57(KIP2) in distinguishing partial mole from its mimics were 88% and 96% respectively(Table 3,4). Regarding the scoring pattern in molar and non-molar pregnancies 92% cases of complete molar abortions showed score 0 and 88% cases of partial mole cases had score 2+ and two cases showed score 1+(score 0 and 1+ is taken as negative expression) and all cases of normal abortions showed score 3+.(Table 5)
Table 1: p57 expression in cytotrophoblast in molar and non-molar abortions
|
P57 expression in cytotrophoblast |
Percentage of cases |
||
|
Complete mole |
Partial mole |
Normal abortions |
|
|
Positive |
4% |
82% |
100% |
|
Negative |
96% |
18% |
0 |
Table 2: p57 expression in villous stromal cells in molar and normal abortions
|
p57 expression in villous stromal cells |
Percentage of cases |
||
|
Complete mole |
Partial mole |
Normal abortions |
|
|
Positive |
8% |
100 |
100 |
|
Negative |
92% |
0 |
0 |
Table 3: Sensitivity and specificity of p57(KIP2) in diagnosing partial moles
|
IHC |
Diagnosis |
sensitivity |
specificity |
PPV |
NPV |
|
P57 |
Partial mole |
88% |
96% |
93% |
92% |
Table 4: The sensitivity and specificity of p57(KIP2) in diagnosing complete mole
|
IHC |
Diagnosis |
sensitivity |
Specificity |
PPV |
NPV |
|
P57 |
Complete mole |
96% |
88% |
92% |
93% |
Table 5: p57 scoring in molar and non-molar abortion specimens
|
P57 scoring |
Percentage of cases |
||
|
Complete mole |
Partial mole |
Normal abortions |
|
|
0 |
92% |
0 |
0 |
|
1+ |
4% |
12% |
0 |
|
2+ |
4% |
88% |
0 |
|
3+ |
0 |
0 |
100% |
Chart 1: Intensity of p57 staining in villous stromal cells in normal abortions
 |
Click here to view |
Chart 2: Intensity of p57 staining in cytotrophoblast in normal abortions
 |
Click here to view |
Chart 3: Intensity of p57 expression in villous stromal cells of partial mole
 |
Click here to view |
Chart 4: Intensity of p57 expression in cytotrophoblast of partial mole
 |
Click here to view |
Chart 5: Intensity of p57 expression in cytotrophoblast and villous stromal cells of complete mole
 |
Click here to view |
The purpose of study was to study the immunohistochemical expression of p57(KIP2) in first trimester abortion specimens of molar and non- molar pregnancies. p57 has shown to be a useful diagnostic tool in diagnosing complete mole when all other tests are equivocal, moreover most institutions cannot afford a DNA ploidy analysis and this marker is a good surrogate in histologically challenging cases. Detailed histopathological examination still remains the basis for the diagnosis of hydatidiform mole (HM).
However, poor sampling, necrosis, and earlier uterine evacuation can always lead to uncertainty in the diagnosis and also the criteria are subjective, resulting in considerable interobserver and intraobserver variability. 100 cases were studied which included 25 cases of complete mole, 17 cases of partial mole and 58 cases of normal abortions. The frequency of complete mole was higher as compared to partial mole similar to the study conducted by Jaffar R et al.[9] In the current study the age group ranged from 18 yrs to 42 yrs and majority of study population were in the age group of 20-30 yrs. Majority of cases diagnosed with complete mole showed circumferential proliferation of syncytiotrophoblast and cytotrophoblast with marked cistern formation, similarly majority of cases diagnosed with partial mole showed focal proliferation of syncytiotrophoblast and cytotrophoblast and moderate to marked cistern formation and normal products did not show any cistern formation or trophoblastic proliferation in line with the study conducted by Jaffir R et al.[9]
Chew S H et al concluded that cistern formation alone cannot make a difference between molar and non- molar gestations, but trophoblastic proliferation is always helpful as polar proliferation favor non-molar abortions.[12] Regarding the expression of p57(KIP2) immunomarker in partial mole diagnosed previously by H&E 88% expressed positive results (score 2+). These findings were highly statistically significant. The results of p57(KIP2) IHC marker expression in cases of complete mole diagnosed previously by H&E, were 24 out of 25 cases which represented 96% and expressed negative results, this confirm the complete mole diagnosis.
The remaining 1 cases which represented 4%, expressed positive result, which means they were most probably partial mole. These findings were statistically highly significant. Among the cases diagnosed with partial mole two cases showed negative staining with p57(KIP2) similar to study conducted by Osterheld M C et al. p57(KIP2) staining was not able to distinguish partial mole from normal abortus comparable to study conducted by Sasaki et al (10) proving that it is a maternally expressed protein product. Normal abortions showed strong expression of p57(KIP2) with a score 3+ in cytotrophoblast and villous stromal cells with syncytiotrophoblast (negative staining),maternal decidua and intermediate villous trophoblast (positive staining) serving as internal control in line with the study conducted by Fukunaga (4).
The sensitivity and specificity of p57 in discriminating complete mole from its mimics were about 96% and 88% respectively similar to study conducted by Russel Vang et al.[13] In the present study the sensitivity and specificity of p57 in discriminating partial mole from its mimics were about 88% and 96% respectively. In the current study there was a positive correlation obtained between the p57(KIP2) score and the histopathological findings proving the fact that as the maternal DNA component is increased the percentage of cells with p57 score is also increased. Thus majority of complete molar cases had score zero, majority of partial mole had score 2+ and all the normal gestations had score 3+ and the concept that the p57(KIP2) is a marker of maternal genome was well documented in a study conducted by Abbas R K et al.[14]
Conclusion
Many studies have proved that even DNA genotyping analysis agreed with the results of p57 immunostaining and p57 is a highly sensitive and specific marker for discriminating complete mole from its mimics in early gestational period. In addition p57 helps in differentiating hydropic abortus from complete mole, a distinction not made by DNA ploidy analysis. Hence p57 immunomarker can be used in concert with DNA ploidy analysis and flow cytometry to refine the diagnosis of early molar geststions.5 But in a developing country like India with limited diagnostic facilities in teritiary care level and financial constrains, p57 can be used as a diagnostic tool equally and totally replacing ploidy analysis to diagnose complete mole and arrive at a diagnosis in histopathologically challenging cases.
Conflict of Interest: None
How to cite : Kumar K P, Jayalakshmy P S, Immunohistochemical expression of p57 (Kip2) in first trimester abortion specimens of molar and non-Molar pregnancies. IP J Diagn Pathol Oncol 2019;4(1):27-31
This is an Open Access (OA) journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Viewed: 3063
PDF Downloaded: 562